Dear Editor,
We read with great interest the recent comprehensive review by Li et al., titled "A Review of the Pathogenesis of IgA Vasculitis Nephritis in Children," published in the Iran Journal of Pediatrics (1). While Li et al. (1) thoroughly describe the pathogenesis of IgA Vasculitis Nephritis (IgAVN), their review does not explore prospective biomarkers such as Pentraxin-3 (PTX-3), highlighting a gap that our study aims to address. We respectfully offer additional insights regarding the critical role of Pentraxin-3, which could further enrich the understanding of IgA Vasculitis Nephritis.
Our prospective cohort study, published in Pediatric Nephrology, demonstrated that elevated serum PTX-3 levels at disease onset significantly predicted subsequent renal involvement in pediatric IgA vasculitis (2). In our study, initial elevated serum PTX-3 levels at disease onset significantly predicted subsequent renal involvement; mean serum PTX-3 was significantly higher in patients who later developed renal involvement compared to patients without renal involvement (2.20 ± 1.30 vs. 1.36 ± 0.85 ng/mL, respectively; P = 0.004) (2). Furthermore, we observed that serum PTX-3 concentrations increased proportionally with the severity of renal involvement classified by Meadow's criteria, providing robust evidence for the biomarker's predictive utility (2).
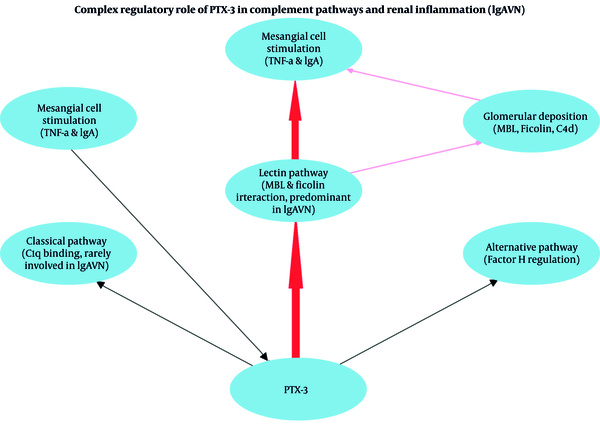
PTX-3 has a complex regulatory role in complement activation, primarily interacting with the lectin pathway components such as mannose-binding lectin (MBL) and ficolins, rather than the classical complement pathway involving C1q (3-7). This distinction is particularly important since previous research highlights that the lectin complement pathway, rather than the classical pathway, plays a pivotal role in the pathogenesis of IgAV nephritis (6). Specifically, Hisano et al. reported activation of the lectin pathway in renal biopsies from patients with Henoch–Schönlein purpura nephritis, emphasizing its significant contribution to renal damage in this condition (6). The interaction of PTX-3 with complement regulators, such as factor H, further supports its role in modulating complement activation and subsequent renal inflammation (5). These mechanisms collectively underscore PTX-3's potential role as an early biomarker for identifying renal involvement risk in IgAV, thus aiding early clinical interventions (Figure 1).
Given the predominantly retrospective nature of previous studies investigating PTX-3 in IgAVN, our prospective findings (2) provide valuable additional evidence that strengthens the clinical applicability of PTX-3 as an early predictor of renal involvement. Incorporating these findings into clinical practice, PTX-3 may enhance clinical strategies for the early detection of renal involvement; however, further validation studies are needed to confirm its practical feasibility in predicting, monitoring, and managing IgAV nephritis.