1. Background
Acinetobacter baumannii is an opportunistic pathogenic bacterium commonly found in soil, water, human skin, and hospital environments (1). It is a frequent contaminant in laboratory settings and plays a significant role in a variety of infections, including bacteremia, meningitis, pneumonia, urinary tract infections, peritonitis, and septicemia. Acinetobacter baumannii is the most common microorganism isolated from blood, sputum, skin, peripheral fluids, urine, and the throat (1, 2). The use of urinary catheters and intravascular catheters often leads to bacteremia and septicemia in burn patients with compromised immune systems (3). Individuals with cystic fibrosis, neutropenia, and immune deficiencies are at higher risk of infection with this bacterium.
A major factor in the widespread nature of A. baumannii is its ability to form biofilms, its high resistance to antibiotics, its capacity to survive both in vivo and in the environment, and its transmission through hospital personnel, equipment, and air (4). In one study, A. baumannii was identified as the most common pathogen in neonatal septicemia (5). Treating infections caused by A. baumannii is challenging due to its significant resistance to a wide range of antibiotics (6). The therapeutic difficulties associated with this pathogen, combined with its potential for transmission, biofilm formation, and persistence in hospital settings, have contributed to its increasing prevalence and ability to cause infections. Biofilm formation is a crucial pathogenic mechanism for many bacteria, allowing them to create protective colonies, resist drugs, and evade host immune responses. Research on A. baumannii biofilm formation has highlighted the role of the bap (biofilm-associated protein) gene in this process (6, 7). Given its high drug resistance, there is a pressing need for new antibacterial agents against this microorganism. Several studies have explored the use of natural medicines, including plant-based remedies, to prevent and treat bacterial infections (8).
Acroptilon repens is a perennial plant with erect stems, growing up to 80 cm tall, found in regions of West Azerbaijan, Zanjan, and Tehran. Known locally as Tarcheh, this plant is used in traditional medicine for its antipyretic properties (9). The stems of A. repens are extensively branched, with solitary flower heads at the end of each branch. Its flowers are urn-shaped and range in color from pink to purple. Acroptilon repens is a noxious weed widely distributed in northwest Iran (10). This plant has allelopathic properties and is toxic to horses. Several studies have highlighted its toxicity, which is attributed to the presence of sesquiterpene lactones and flavones, such as 7,8-benzoflavone, secreted from its roots. The plant has been referred to in the literature as Centaura repens (9).
2. Objectives
The present study investigates the effects of A. repens extract, along with its chloroform, ethyl acetate, and water fractions, on inhibiting the growth of A. baumannii, reducing biofilm formation, and modulating the expression of the bap gene in A. baumannii.
3. Methods
3.1. Plant Material and Bacteria
The A. repens plant was collected from the farms of Maragheh city (west of Iran) during the flowering stages in spring and autumn. The aerial parts of the plant were selected for extraction and fractionation. Botanical identification was carried out at the Herbarium of the Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran, with voucher No: Tbz-Fph-4041.
In this study, the standard strain A. baumannii ATCC25923, obtained from the Iranian Research Organization for Science and Technology (IROST) (11), was used to assess antibacterial, antibiofilm, and bap gene expression.
3.2. Extraction and Fractionation of Acroptilon repens
For extraction, the aerial parts of A. repens, which had been dried in the shade, were ground into a powder at room temperature. A total of 100 grams of powdered A. repens was weighed and placed in a container. Ethanol was then added to cover the powder. After 24 hours, the sample in the Erlenmeyer flask was filtered using filter paper and a sieve. The solution was stored in a dark place away from light. Extraction was then carried out by vacuum distillation using a rotary evaporator at a temperature of 40°C and a speed of 88 rpm for four hours. The concentrated extract was transferred to a sterile glass container, covered with foil, and stored in a freezer at -20°C. From every 100 grams of A. repens powder, 3.4 grams of ethanolic extract were obtained (12).
To prepare the fractions, 5 grams of the ethanolic extract were diluted in 75 milliliters of distilled water. The solution was poured into a decanter. For the chloroform fraction, 15 milliliters of chloroform were added to the solution. After mixing and separating into two phases, the chloroform phase was collected. This process was repeated five times, and the chloroform phase was concentrated using a rotary evaporator. In this way, the chloroform fraction was separated. The ethyl acetate fraction was obtained by adding ethyl acetate using a similar method. After evaporating the water, the remaining solution was stored as the aqueous fraction (13).
3.3. Antibacterial Activity Assessment
The antibacterial effects of the A. repens extract and its fractions were evaluated using three methods:
3.3.1. Disk Diffusion Method
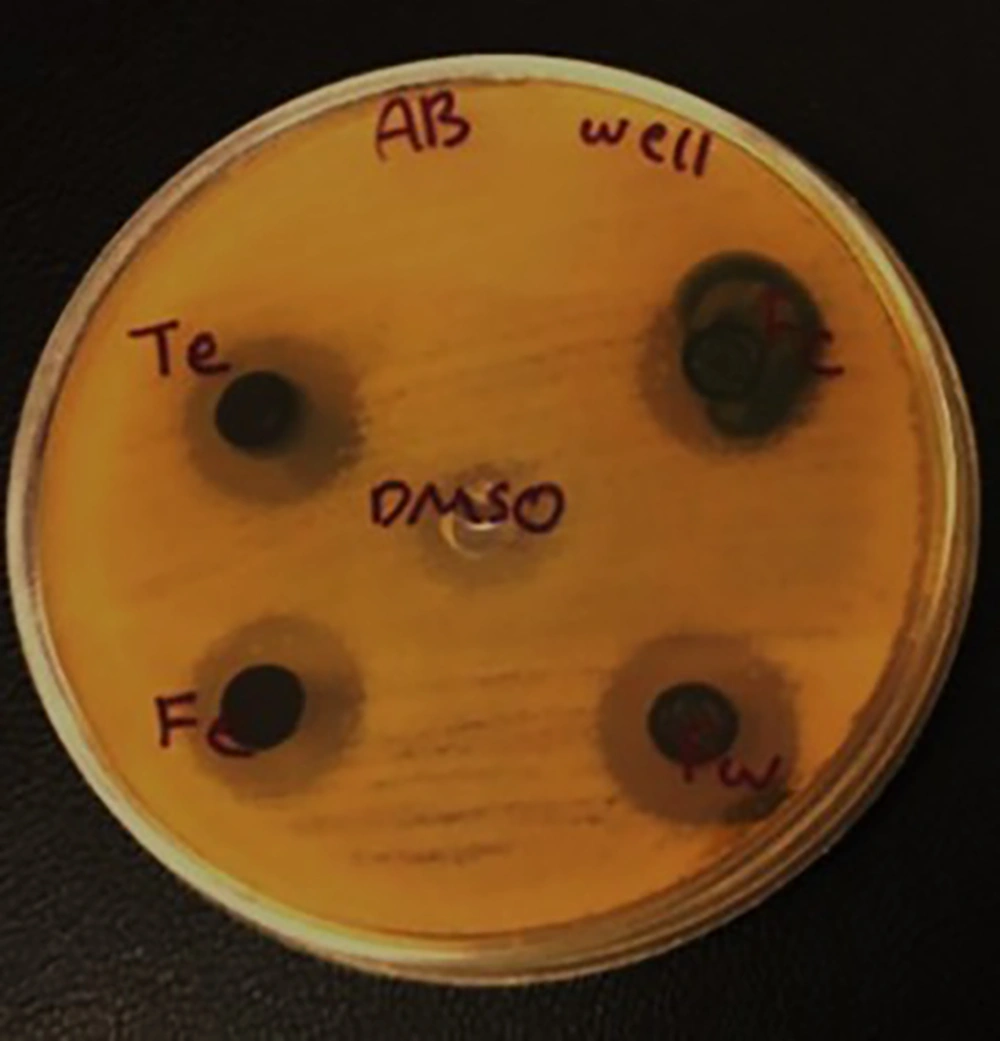
An equivalent of a half McFarland suspension was initially prepared from A. baumannii in PBS. A sterile swab was immersed in the microbial suspension, and the excess solution was removed by pressing the swab against the side of the tube. The swab was then cultured on a Mueller-Hinton agar plate. Under sterile conditions, four sterile blank discs were placed on the plate using sterile forceps. Using a micropipette, 50 microliters of the ethanolic extract and its chloroform, ethyl acetate, and aqueous fractions were applied to the discs. The plate was incubated at 37°C for 24 hours, and the growth inhibition zone around the discs was measured. A disc soaked in DMSO solvent was used as a negative control (14).
3.3.2. Well Diffusion Method
The A. baumannii culture was prepared in the same manner as the disk diffusion method. Four wells of 2 millimeters were made in each plate using a Pasteur pipette. Then, 100 microliters of the ethanolic extract and its chloroform, ethyl acetate, and aqueous fractions were added to each well using a micropipette. The plate was incubated at 37°C for 24 hours, and the results were recorded (14).
3.3.3. Minimum Inhibitory Concentration and Maximum Bactericidal Concentration Determination
The broth dilution method was used to determine the minimum inhibitory concentration (MIC) and maximum bactericidal concentration (MBC) of the ethanolic extract and the chloroform, ethyl acetate, and aqueous fractions of A. repens against A. baumannii. Different concentrations of the fractions were prepared in the culture medium, as outlined in Tables 1 and 2. Subsequently, 10 microliters of the microbial suspension, equivalent to half McFarland (1.5 × 108 CFU/mL), were added to each tube. The tubes were then placed in a shaker incubator at 37°C for 24 hours. After incubation, 10 microliters from each tube were transferred to a Mueller-Hinton agar plate, spread using a sterile loop, and incubated at 37°C for 24 hours. The MIC and MBC were determined by counting the colonies on the agar plates (15).
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Acroptilon repens extract or its fractions (mg) | 125 | 62 | 31 | 15.5 | 7.75 | 3.9 | 1.9 |
| Culture medium (mL) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Concentration (mg/mL) | 250 | 124 | 62 | 31 | 15.5 | 5.75 | 3.9 |
Tube Compositions in Determining Minimum Inhibitory Concentration and Maximum Bactericidal Concentration of Ethanol Extract and Chloroform and Ethyl Acetate Fractions
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Acroptilon repens extract or its fractions (mg) | 10 | 5 | 2.5 | 1.25 | 0.62 | 0.31 | 0.15 |
| Culture medium (mL) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Concentration (mg/mL) | 5 | 2.5 | 1.25 | 0.62 | 0.31 | 0.15 | 0.075 |
The Composition of the Tubes in Determining the Minimum Inhibitory Concentration and Maximum Bactericidal Concentration of the Aqueous Fraction
3.4. Antibiofilm Activity Assay
To assess the antibiofilm activity, 250 microliters of nutrient broth culture medium containing a sub-MIC concentration of the ethanolic extract, chloroform, ethyl acetate fractions, and water fraction of A. repens were added to the wells of a sterile microtiter plate. Next, 10 microliters of A. baumannii suspension, equivalent to 0.5 McFarland turbidity (1.5 × 106 CFU), were added to each well. The culture medium with and without bacteria served as the two control wells. The plate was incubated at 37°C for 24 hours.
After incubation, the contents of the wells were discarded, and the wells were dried. The wells were washed three times with PBS, and the plate was vigorously shaken to remove unattached cells. Subsequently, 200 microliters of diluted fuchsine were added to the wells. After 2 minutes, the contents of the wells were discarded and washed once with PBS. Then, 200 microliters of acetone alcohol were added to the wells, and the plate was shaken for 5 minutes.
The optical density (OD) of the wells stained with diluted fuchsine was measured at a wavelength of 492 nm using an ELISA reader. The following formula was used to calculate the antibiofilm activity of the extract and fractions (16):
3.5. Effects of Acroptilon repens on bap Gene Expression
In this step, A. baumannii was treated with sub-MIC concentrations of A. repens extract and its fractions. RNA extraction was performed according to the instructions of the RNX-Plus extraction kit. The extracted RNA was then used for cDNA synthesis using the SINACLON RT5202 kit. The cDNA samples (from both treatment and control groups) were subsequently used for real-time PCR analysis.
For the real-time PCR reaction, 12.5 microliters of SYBR Green Mastermix, along with 0.5 microliters of forward (F) primers and 0.5 microliters of reverse (R) primers, were added to each of the PCR tubes. Then, 7.5 microliters of deionized water and 4 microliters of synthesized cDNAs (from both the treatment and control groups) were added to each tube. The tubes were then placed into the real-time PCR machine. The temperature conditions and primer details used for the PCR are shown in Table 3.
| Genes | Primers | Product Size (bp) | |
|---|---|---|---|
| bap | 183 | ||
| Forward | 5'-TGCTGACAGTGACGTAGAACCACA-3' | ||
| Reverse | 5'- TGCAACTAGTGGAATAGCAGCCCA-3' | ||
| DNA gyrase A | 110 | ||
| Forward | 5’-AAGGCCGTCCAATCGTGAA<T>-3’ | ||
| Reverse | 5’-AACCGTACCAGAAGCTGTC<G>-3' | ||
| Stage | Temperature (°C) | Time (s) | Cycle (No.) |
| Initial denaturation | 95 | 120 | 1 |
| Denaturation | 93 | 45 | 35 |
| Annealing | 57 | 60 | |
| Extension | 72 | 30 | |
| Final extension | 72 | 90 | 1 |
Primers and Thermocycling Conditions for bap Gene Amplification
3.6. Data Analysis
Data analysis was performed using SPSS software. The quantitative results were statistically analyzed based on the average of three repetitions. The data were evaluated using the ANOVA test and the independent t-test for comparison.
4. Results
4.1. Antibacterial Activity
The results showed that the extract of A. repens and its fractions exhibited antibacterial activity against A. baumannii. This antibacterial effect was observed across all three methods: Disk diffusion, well diffusion, and determination of MIC and MBC. Overall, the antibacterial effect of the A. repens extract was greater than that of its fractions. The detailed results of the antibacterial activity of A. repens extract and its fractions are presented in Table 4 and Figure 1.
| Bacteria | Ethnol Extract | Chlorophorm Fraction | Ethil Acetate Fraction | Water Fraction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disk | MIC | MBC | Well | Disk | MIC | MBC | Well | Disk | MIC | MBC | Well | Disk | MIC | MBC | Well | |
| Acinetobacter baumannii inhibition zone | 10mm | 31.2 µg/mL | 62.5 µg/mL | 15Mm | 9Mm | 7.8 µg/mL | 15.6 µg/mL | 15Mm | 9Mm | 7.8 µg/mL | 15.6 µg/mL | 15mm | 8mm | 0.62 µg/mL | 1.25 µg/mL | 16mm |
Antibacterial Activity of the Extract and Fractions of Acroptilon repens Against Acinetobacter baumannii
The antibacterial activity of the ethanolic extract of A. repens was compared with that of antibiotics such as imipenem, ciprofloxacin, and chloramphenicol against A. baumannii using the disk diffusion method. The extract showed acceptable results in comparison (Table 5).
The Antibacterial Effect of Ethanolic Extract of Acroptilon repens Comparison with Imipenem, Ciprofloxacin and Chloramphenicol
4.2. Anti-biofilm Activity
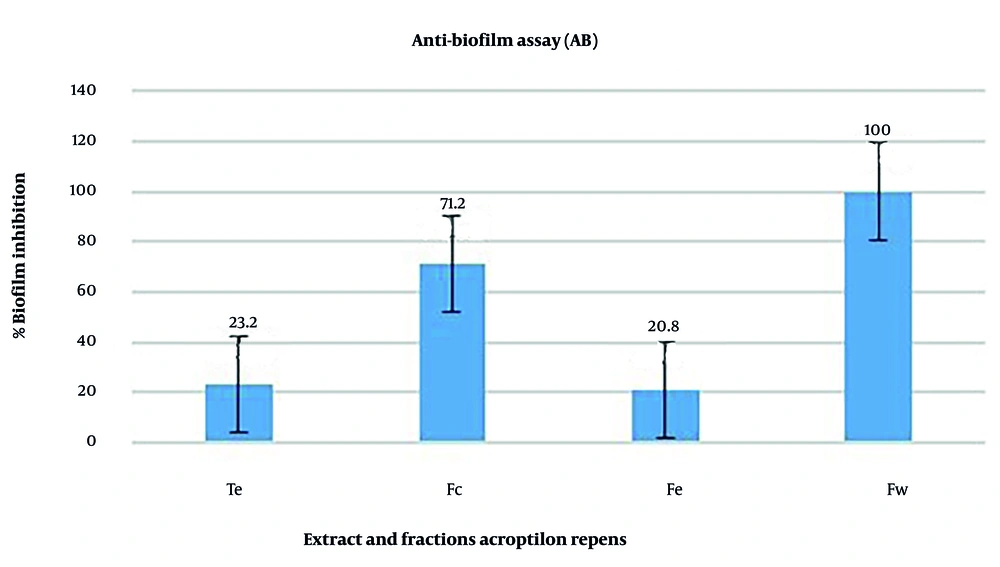
The anti-biofilm activity results against A. baumannii are presented in Figure 2. The results showed that biofilm formation in A. baumannii was inhibited by the ethanolic extract and fractions of A. repens. Among these, the ethanolic extract and ethyl acetate fraction exhibited the least anti-biofilm effect, while the chloroform and aqueous fractions demonstrated the most significant anti-biofilm activity against A. baumannii. As shown in Figure 2, the percentage of anti-biofilm activity for the ethanolic extract, and the chloroform, ethyl acetate, and aqueous fractions were 23.2%, 71.2%, 20.8%, and 100%, respectively.
4.3. Effect of Acroptilon repens Extract and Its Fractions on bap Gene Expression
The study results on the expression of the bap gene before and after treatment with sub-MIC concentrations of the ethanolic extract and fractions of A. repens, relative to the reference gene DNA gyrase A of A. baumannii, are shown in Figure 3.
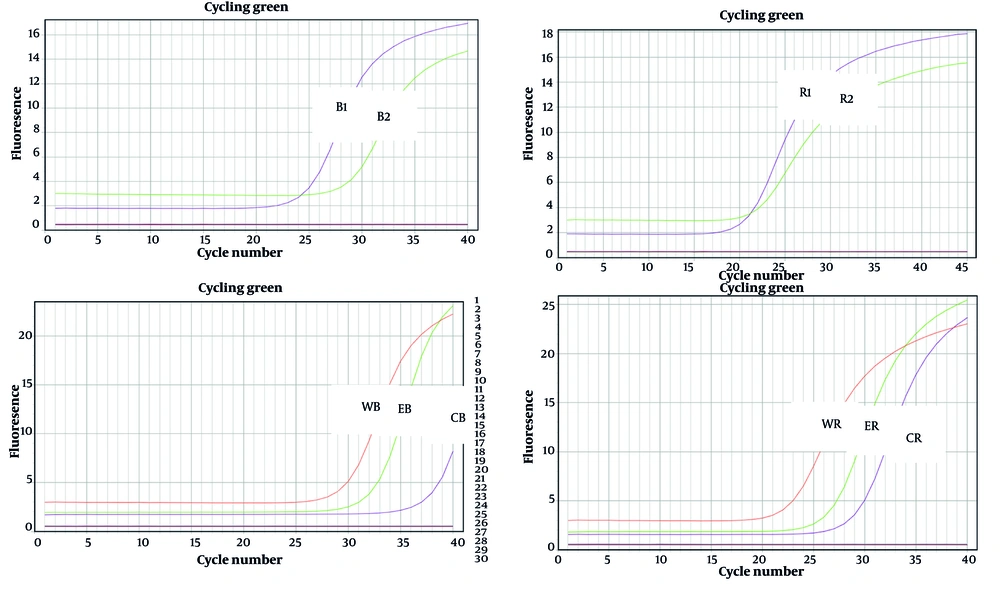
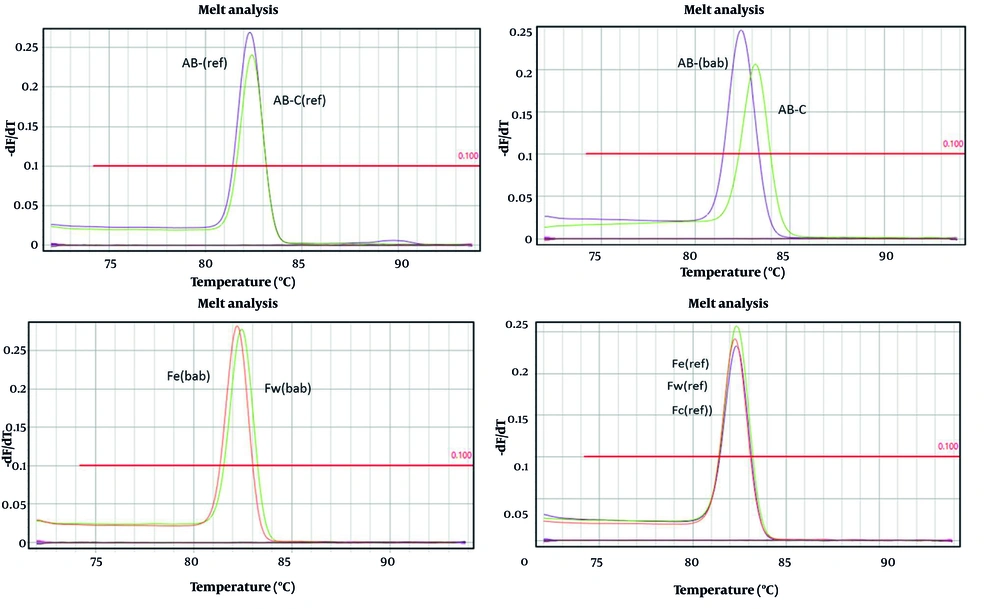
A melting curve was plotted to confirm the specific amplification of genes, particularly housekeeping genes. As shown in Figure 4, the melting curve for the bap gene was generated, and the presence of a sharp peak indicates that there was no primer dimer, the amplification was specific, and the results were accurate.
After obtaining the average CTs, they were analysed using the formula below.
Calculations related to bap gene expression:
Ratio (Fc) = 27.07 - 30.98/21.04 - 20.38 = -3/91/0.66 = -5.92
C T = 25.92 = 60.54
Ratio (Fe) = 27.07 - 29.99/21.04 - 20.43 = -2.92/0.61 = -4.7
C T = 24.7 = 25.99
Ratio (Fw) = 27.07 - 29.02/21.04 - 20.58 = -1.95/0.46 = -4.2
C T = 24.2 = 18.37
Ratio (Te) = 27.07 - 23.1/21.04 - 20.57 = 3.97/0.47 = 8.4
C T = 2-8.4 = 0.0029
5. Discussion
Considering the increasing prevalence of antibiotic resistance among pathogenic bacteria, replacing conventional drugs with natural substances such as plant-derived compounds is emerging as one of the most promising strategies for treating infections caused by resistant bacteria (17). Furthermore, due to the high resistance of A. baumannii to phenols, phenolic compounds, drugs, and disinfectants, as well as its significant role in nosocomial infections and infections in burn and immunocompromised patients, finding an effective and safe therapeutic alternative is crucial. Medicinal plants are of significant importance due to their therapeutic phytochemical compounds, which hold potential for the development of new drugs (18).
Herbal extracts can act through various mechanisms. In this study, the antibacterial and anti-biofilm activities of the ethanolic extract, chloroform fraction, and ethyl acetate fraction of the aerial parts of A. repens were evaluated against A. baumannii. The antibacterial activity of A. repens was assessed using disk diffusion, well diffusion, and MIC-MBC determination methods. Based on the results, the ethanolic extract, chloroform fraction, ethyl acetate fraction, and aqueous fraction of A. repens demonstrated clear antimicrobial effects. Water was used as the solvent in this study; however, DMSO was used when the fractions were insoluble in water (0.1%). Approximately 10%, 20%, and 40% of the fractions dissolved completely in water, while the remainder was dissolved using the maximum permissible DMSO concentration in biological systems.
In a study by Norouzi-Arasi et al., the extract of the aerial parts of A. repens exhibited strong antimicrobial effects against P. aeruginosa (9). A similar result was observed for A. baumannii in our study. One of the most critical factors in the pathogenicity of A. baumannii is its ability to form biofilms. Eliminating biofilm formation can significantly reduce bacterial pathogenicity. This study evaluated the effect of A. repens extract on biofilm formation, and the results showed remarkable anti-biofilm potential of A. repens and its fractions against A. baumannii. The ethanolic extract, chloroform, ethyl acetate, and aqueous fractions exhibited varying degrees of anti-biofilm activity, with the chloroform and aqueous fractions showing the most significant effects.
Alejandro et al. reported that the methanolic extract of Nivaloscordum bivalve contains secondary metabolites with antimicrobial and anti-biofilm properties against A. baumannii. The active compounds in this extract, at sub-MBC concentrations, disrupted biofilm stability and altered bacterial morphology, similar to our findings with A. repens on A. baumannii (19). The chemical composition of the ethanolic extract of A. repens was studied by Zhao et al. using chromatography. Eleven compounds were isolated and identified, including 2alpha, 9beta-dihydroxy-dehydrocostus lactone, cynaropicrin, apigenin, stigmasterol, 4'-hydroxywogonin, ethyl caffeate, p-methoxy-cinnamic acid, luteolin, daucosterol, apigenin-7-O-beta-D-glucoside, and syringin (20).
Additionally, the study by Dahham et al. demonstrated that β-caryophyllene exhibited more potent antimicrobial activity than kanamycin against Staphylococcus aureus, along with antifungal properties (21). The results from this study indicate that the extract and fractions of A. repens are capable of inhibiting bacterial growth and reducing bacterial pathogenicity without significantly affecting the expression of bacterial virulence factors. This suggests that compounds in A. repens may hinder bacterial growth through multiple mechanisms, such as damaging the cytoplasmic membrane, cell wall, secretion systems, and efflux pumps. As a result, the likelihood of bacterial resistance to such compounds is relatively low. Considering the acceptable antibacterial and anti-biofilm effects of A. repens extract and its fractions against A. baumannii and other bacteria — especially with their potential for targeting multiple mechanisms and low resistance emergence — A. repens appears to be a promising natural source of antibacterial agents.
However, further studies are required to validate and expand upon the findings of this study, particularly in the following areas: (1) Investigating the antibacterial and anti-biofilm effects of A. repens extract and its fractions against a broader spectrum of gram-negative, gram-positive, and drug-resistant bacteria.; (2) examining the cytotoxic effects of A. repens extract and its fractions on various cancerous and non-cancerous cell lines; (3) evaluating the antibacterial effects of A. repens extract and its fractions in an infection animal model; (4) investigating the antibacterial and cytotoxic mechanisms of A. repens extract and its fractions on gram-negative and gram-positive bacteria separately; (5) assessing the antibacterial and anti-biofilm effects of A. repens extract and its fractions from different plant parts (aerial parts, roots, and flowers); (6) comparing the antibacterial and anti-biofilm effects of A. repens extract and its fractions derived from plants grown in different geographical areas.