1. Context
In late 2019, an atypical respiratory infection emerged in Wuhan, China, caused by a novel coronavirus (SARS-CoV-2) (1). This led to the devastating coronavirus (COVID-19) pandemic, posing a critical threat to global health, with incidence rates varying widely — from 61.4 cases per 1,000,000 people in South Korea to just 0.0002 in India (2-4). The incubation period is approximately 5 to 6 days (5). Common symptoms include fever, cough, dyspnea, malaise, and fatigue (6). Additionally, the potential for multi-organ involvement during the illness can lead to death (7). Beyond its high fatality rate, COVID-19 can cause significant psychological issues and long-term morbidities (8-10). Despite numerous treatment options and the development of COVID-19 vaccines, scientists continue to seek effective treatments (11).
Traditional and herbal medicines are low-cost options for disease prevention and treatment, widely available and popular in many countries (12). Studies suggest the potential antiviral effects of various herbal plants, such as Echinacea and Curcumin, which may be applicable for these purposes (13, 14). The experience of using Chinese herbal medicine for Middle East respiratory syndrome (MERS), 2003 SARS, and 2009 H1N1 influenza (15) prompted many governments, including China and India, to integrate traditional medicine with routine COVID-19 patient care and investigate its effects on disease outcomes (2, 16).
Evidence indicates that traditional therapies can be effective in treating COVID-19. Zingiber and Echinacea improved cough and dyspnea but did not significantly reduce hospitalization rates. A traditional Chinese medicine demonstrated faster fever resolution and lower acute respiratory distress syndrome (ARDS) development. A polyherbal formulation from India’s Siddha system of medicine reduced viral load without symptom progression, while curcumin/piperine enhanced recovery and reduced hospitalization duration (12, 17-19). However, limited data on safety, adverse effects, and specific dosing for each herbal plant in managing COVID-19 have been noted (13). These points underscore the need for further studies to clarify these ambiguous aspects.
The aim of this study was to systematically review the related literature and perform a meta-analysis on the effects of traditional/herbal medicine in various aspects, including prophylaxis, treatment, and outcomes of patients with COVID-19.
2. Evidence Acquisition
To evaluate the effectiveness of herbal medicine add-on treatments for COVID-19, a systematic review with meta-analysis of randomized controlled trials (RCTs) was conducted.
2.1. Search Strategy and Data Sources
We searched PubMed, Scopus, Embase, Web of Knowledge, and Google Scholar to identify relevant articles, using search queries for "title or abstract" from January 2019 to January 2024, with English language restrictions. Search queries are provided in Appendix 1 in Supplementary File. Additionally, a supplementary search was manually performed by tracing the references of systematic reviews and meta-analyses. Articles were listed and managed using Mendeley.
2.2. Inclusion/Exclusion Criteria
Studies were included if they met the following criteria: (1) The study type was a RCT; (2) included adults diagnosed with COVID-19 confirmed by RT-PCR test; (3) compared the effectiveness of herbal medicine add-on treatment to the standard of care (SOC), with a control group receiving either only SOC or SOC plus placebo; (4) data for at least one of the primary outcomes (i.e., COVID-19 symptom improvement rate, symptom-free rate, or symptom recovery rate) or secondary outcomes [i.e., hospital length of stay (LoS) duration decline, deterioration or mortality rate, and RT-PCR negative conversion rate] were reported or could be retrieved; (5) follow-up lasted for at least 7 days from the day after the last dose of intervention was received.
Studies were excluded for the following reasons: (1) The index day of the study was the day that signs/symptoms appeared instead of the randomization day; (2) outcome measures for effectiveness were not clearly defined; (3) target outcomes could not be extracted in a way that at least one outcome of dichotomous or continuous variable could not be extracted or retrieved, even with approximation transforming formulas; (4) suspected COVID-19 subjects were included; (5) study design was non-randomized trial, prospective cohort, or retrospective cohort; (6) full text was not available; or (7) it was not written in English. The population, intervention, comparison, and outcome characteristics of the study are provided in Appendix 2 in Supplementary File.
2.3. Study Selection
To ensure reproducibility, two independent researchers (GH, MFK) independently screened the initial list obtained from the search, read the full text of eligible studies (second screening), extracted the target data, and evaluated the risk of bias (RoB) for the studies included in the quantitative analysis. Disagreements in these processes were resolved through discussion, and consensus was reached in all cases.
2.4. Data Extraction
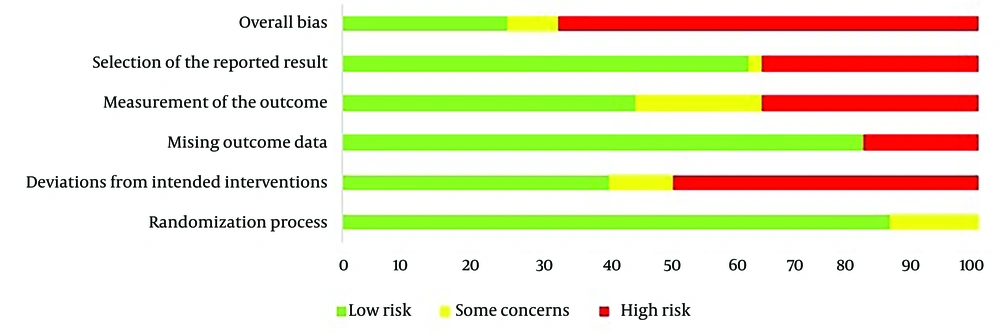
The updated Cochrane collaboration tool for RoB 2 was used for quality assessment, and studies included in the quantitative analysis were scored for (1) overall bias, (2) selection of the reported result, (3) measurement of the outcome, (4) missing outcome data, (5) deviations from intended interventions, and (6) randomization process. Each study was ranked as "low risk", "some concerns", or "high risk" for these items. Details on RoB 2 can be found elsewhere (20).
A spreadsheet was prepared to include all targeted information and statistics for the studies included in the quantitative analysis. This checklist included: General data (first author’s name, publication year, and country) and group-specific data [study design, setting, sample size (total, groups), total and group characteristics (sex, age), study duration (intervention, follow-up), WHO Ordinal Severity Scale, critical exclusion criteria (a nested checklist, Appendix 3 in Supplementary File), brief description of intervention (dose, interval), outcomes, and assessing RoB 2. Outcomes included overall and sign/symptom [fever, cough, and dyspnea (shortness of breath)] improvement rate, deterioration rate, time needed for recovery, RT-PCR conversion rate, time needed for RT-PCR conversion, and hospital LoS.
Due to the diversity in nomenclature, we considered terms such as "need to receive high flow oxygen therapy", "need for ventilator machine", "need to transfer to intensive care unit (ICU)", "need to hospitalize", "turn to ARDS" or "critically ill", "worsening of condition", "deterioration of condition", etc., as equivalent (index outcome = deterioration rate). Similarly, terms like "improvement", "recovery", "symptom-free rate", "without symptoms", "significant improvement of symptoms", "excellent or good results", "effective rate", "overall change in symptom", "therapeutic effect", etc., were considered equivalent (index outcome = improvement rate). Outcomes were extracted using the intention-to-treat (ITT) method.
2.5. Statistical Analysis
Statistical analysis was conducted using RevMan software (Cochrane Collaboration; version 5.4.1; released September 2020). Dichotomous and continuous outcomes were reported as risk ratio (RR) with 95% confidence interval (CI) and standardized mean difference (SMD) with 95% CI, respectively. Forest plots were constructed to visualize the effect sizes (with 95% CIs) of studies, as well as the calculated summary effect size (with 95% CI).
To assess heterogeneity (between-study variance; tau²) around the summary effect size, we performed a χ² test (general heterogeneity) and calculated the I² statistic (the proportion of heterogeneity attributable to between-study variance). An I² greater than 50% or P < 0.1 was considered significant for heterogeneity. The Z(u) test was used for hypothesis testing of group comparisons, with a P-value < 0.05 indicating statistical significance between groups.
Funnel plots were used to assess publication bias for main outcomes. While both the Begg and Egger tests are important, we relied on the funnel plot for our analysis due to the limited number of studies included in our review.
We encountered several considerations regarding data extraction and analysis: First, random-effects models were used to calculate summary effect size due to high heterogeneity. Second, sensitivity analysis was employed to address high heterogeneity; summary effect sizes were recalculated after removing outlying studies. Third, for quantitative outcomes measured differently, we used the SMD instead of the mean difference (MD) as a summary statistic. Where necessary, we applied the formula proposed by Wan et al. (21) to transform "the first quartile, median, the third quartile, and sample size" into "the estimated mean and the estimated standard deviation (SD)".
2.6. Ethics Approval
This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1399.629). Additionally, the study protocol is registered with the Center for Open Science (https://osf.io/kmrwd).
3. Results
3.1. Description of Included Studies
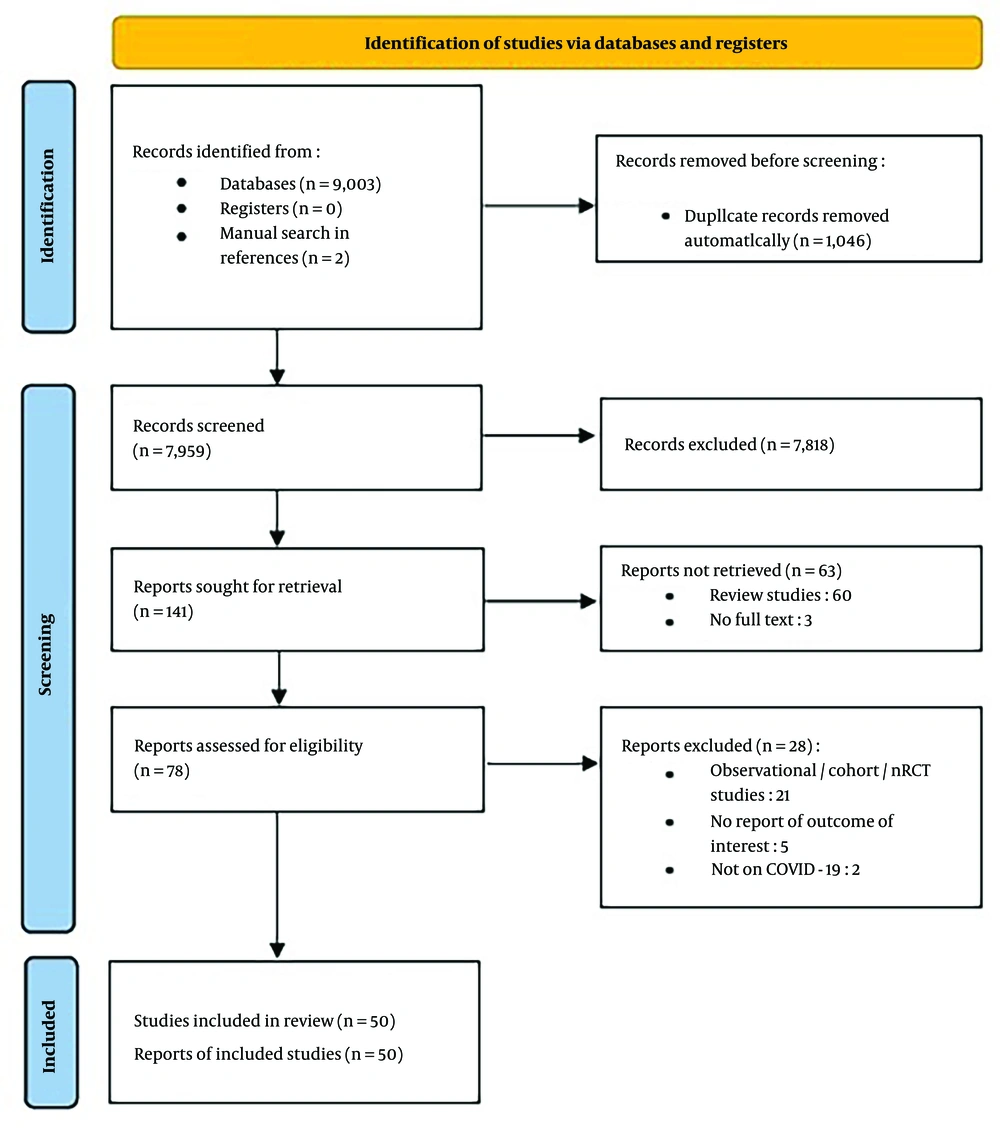
We identified 9,003 records through database searching. After removing duplicates, 7,957 studies remained, along with two studies that were manually added, resulting in a total of 7,959 studies for title and abstract review. Of these, 141 records were eligible for full-text review, yielding 78 studies for qualitative synthesis. Finally, 50 RCTs (2, 12, 16, 17, 19, 22-66) were included in the quantitative synthesis (meta-analysis). The reasons for exclusion are shown in the flowchart of included studies (Figure 1).
The total sample size was 6,031, comprising 3,184 patients in the intervention group and 2,847 patients in the control group. The number of participants in each study ranged from 42 to 408. Most studies were conducted on patients with mild-to-moderate COVID-19 (WHO Ordinal Severity Scale of I to V), either hospitalized or outpatient. Seven studies included patients with severe or critical COVID-19 (WHO Ordinal Severity Scale of V-VII). Characteristics of the included studies are shown in Table 1. Additionally, the RoB graph is shown in Figure 2.
| Authors | Study Design | Setting | Sample Size (Experimental-Control) | Study Duration | Sample Size (Experimental - Control), Gender Stratified (Male-Female) | Age (Control, Experimental) | Country | Declared Clinical Score of Participants | Most Probable Clinical Score of Included Participants | Regimen (Name, Interval, Duration) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | ||||||||||
| Gupta et al. (2021), (22) | R- OL | M (30) | 35 - 33 | 28 | 19 - 16, 24 - 9 | 39.20 ± 11.68; 41.06 ± 12.12 | India | Mild-to-moderate | I-III | Ayurveda BID + SOC for 28 days | SOC |
| Wanjarkhedkar et al. (2020), (23) | OL | S | 60 - 39 | 7 | NA | 44.03 ± 11.78; 41.59 ± 13.6 | India | (Hospitalized) mild-to-moderate | III, IV | Ayurveda BID + SOC for 7 days | SOC |
| Liu et al. (2021), (2) | R-OL | S | 99 - 96 | 15 - 17 | 36 - 63, 37 - 59 | 56 (48.50 - 62); 56.5 (48.75 - 62.25) | China | Mild | I-IV | Q-14 BID + SOC for 14 days | SOC |
| Mesri et al. (2021), (12) | RCT | S | 50 - 50 | 14 | 31 - 19, 29 - 21 | 47.1 ± 15.53; 45.46 ± 13.46 | Iran | Mild | I, II | Zingiber officinale + echinacea + SOC for 7 days | SOC |
| Wang et al. (2020), (17) | RCT | M | 24 - 23 | 28 | 14 - 10, 12 - 11 | 46.8 ± 14.4; 51.4 ± 17.6 | China | Mild | I-IV | Keguan-1 BID + SOC | SOC |
| Ni et al. (2021), (24) | R-OL | M | 56 - 61 - 59 - 59 | 14 | 23 - 33 (low dose), 33 - 28 (mid dose), 27 - 32 (high dose), 25 - 31 | 54 (42 - 62.25); 56 (44 - 65); 53 (41.5 - 63); 51 (38.5 - 65) | China | Mild-to-moderate | I-IV | Shuanghuanglian TID + SOC for 14 days | SOC |
| Srivastav et al. (2021), (16) | RCT | M | 40 - 40 | 40 | 20 - 20, 20 - 20 | 39.5 (NA); 44.4 (NA) | India | Mild-to-moderate | I-IV | Nilavembu Kudineer (NVK) BID + SOC for 10 days | SOC |
| Majeed et al. (2021), (25) | RCT | M | 45 - 47 | 28 | 71 - 29 | 39.04 ± 7.70; 37.28 ± 7.40 | India | Mild-to-moderate | I-III | ImmuActiveTM QD + SOC | Placebo QD + SOC |
| Xu et al. (2021), (26) | R-OL | M | 77 - 77 | 28 | 43 - 34, 44 - 36 | 49.1 ± 15.7; 50.4 ± 16.0 | China | Mild-to-moderate | I-III | IV Reduning QD + SOC for 14 days | SOC |
| Zhao et al. (2021), (27) | Unblinded RCT | S | 204 - 204 | 28 | 88 - 116, 94 - 110 | 52.0 (39.3 - 60.8); 50.0 (39.0 - 59.3) | China | Mild | I-III | Huashibaidu granule BID + SOC for at-least 7 days | SOC |
| Pawar et al. (2021), (19) | RCT | S | 55 - 55 | 14 | 38 - 17, 43 - 12 | NA | India | Mild-to-moderate | I-IV | Curcumin C3 Complex® BID + SOC for 14 days | SOC |
| Di Pierro et al. (2021), (28) | R-OL | S | 76 - 76 | 30 | 42 - 34, 46 - 30 | NA | Pakistan | Mild | I, II | Querceti formulated with sunflower lecithin BID + SOC for 30 days | SOC |
| Sardari et al. (2021), (67) | NA | S | 40 - 43 | 7 | 21 - 19, 14 - 29 | 43 (26); 58 (24) | Iran | (Hospitalized) mild-to-moderate | III, IV | thyme essential oil TID + SOC for 7 days | SOC |
| Zhang et al. (2021), (30) | R-OL | M | 65 - 65 | 28 | 32 - 33, 28 - 37 | 44.31 ± 13.45; 48.25 ± 14.22 | China | (Hospitalized) mild-to-moderate | III | XYP injection + SOC for 7-14 days | SOC |
| Karimi et al. (2021), (31) | R-OL | M | 184 - 174 | 7 | 106 - 76, 91 - 85 | 48.72 ± 14.863; 50.79 ± 15.878 | Iran | (Hospitalized)moderate | III, IV | TPM polyherbal decoction TID + two herbal capsules BID + SOC for 7 days | SOC |
| Devpura et al. (2021), (32) | pilot RCT | S | 45 - 50 | 7 | 35 - 10, 42 - 8 | 33.40 ± 9.4; 35.4 ± 10.4 | India | Mild | I, II | ayurvedic regime BID + SOC for 7 days | Placebo BID + SOC for 7 days |
| Hu et al. (2021), (33) | R-OL | M | 142 - 142 | 14 | 79 - 63, 71 - 71 | 50.4 ± 15.2; 51.8 ± 14.8 | China | Mild-to-moderate | I-IV | Lianhuaqingwen TID + SOC for 14 days | SOC |
| Koshak et al. (2021), (34) | R-OL | S | 91 - 92 | 14 | 48 - 43, 49 - 43 | 35 ± 10; 36 ± 12 | Saudi Arabia | Mild | I-III | NSO (MARNYS® cuminmar) BID + SOC for 10 days | SOC |
| Ma et al. (2021), (35) | R-OL | M | 27 - 23 | 14 | 16 - 11, 12 - 11 | 49.7 ± 16.0; 51.5 ± 15.9 | China | (Hospitalized) mild-to-moderate | III, IV | IV RDN QD + SOC for 14 day | SOC |
| Xiao et al. (2020), (36) | Unblinded RCT | S | 61 - 63 | 14 | 33 - 28, 35 - 30 | 54.31 ± 11.63; 54.06 ± 13.90 | China | Mild | I, II | Huoxiang BID + lianhua TID + SOC | SOC |
| Zeng et al. (2021), (37) | R-OL | S | 29 - 30 | 14 | 19 - 11, 21 - 8 | 50.7 ± 12.3; 53.3 ± 15.8 | China | Mild-to-moderate | I-IV | MWD BID + SOC for 14 days | SOC |
| Zhou et al. (2021), (38) | R-OL | M | 57 - 54 | 14 | 33 - 24, 38 - 16 | NA | China | Severe or critical | V-VII | SHG BID + SOC for at-least 14 days | SOC |
| Setayesh et al. (2022), (39) | R-OL | S | 38 - 41 | 14 | 21 - 17, 22 - 19 | 59.1 ± 17.1; 59.2 ± 17.2 | Iran | (Hospitalized) mild-to-moderate | III-V | TPM + SOC TID + SOC for 7 days | SOC |
| Tavakoli et al. (2022), (40) | RCT | S | 48 - 49 | 14 | 37 - 12, 29 - 19 | 56.8 ± 13.5; 50.2 ± 13.8 | Iran | (Hospitalized) mild-to-moderate | III, IV | PBW QD + SOC for 14 days | SOC |
| Adel Mehraban et al. (2023), (41) | RCT | S | 54 - 54 | 7 | 27 - 27, 28 - 26 | NA | Iran | Mild | I | Violet syrup + brown sugar + SOC for 7 days | Placebo + SOC |
| Ahmadpour et al. (2023), (42) | RCT | S | 48 - 46 - 47 | 5 | 24 - 24, 22 - 24, 23 - 24 | 49.54 ± 12.72; 50.44 ± 11.91 | Iran | (Hospitalized) mild-to-moderate | III, IV | 250 or 500 mg olive leaf extract BID + SOC for five days | Placebo + SOC |
| Asadirad et al. (2022), (43) | RCT | S | 30 - 30 | 7 | 24 - 6, 24 - 6 | 56 ± 14.02; 50.2 ± 12.01 | Iran | (Hospitalized) mild-to-moderate | III, IV | 40 mg curcumin QID + SOC for 7 days | Placebo + SOC |
| Askari et al. (2022), (44) | RCT | S | 23 - 23 | 14 | 14 - 9, 13 - 10 | 43.74 ± 12.9; 51.52 ± 13.8 | Iran | Mild-to-moderate | III, IV | 500 mg curcumin + 5 mg piperine + SOC for 14 days | Placebo + SOC |
| Borujerdi et al. (2022), (45) | RCT | S | 59 - 57 | 10 | 33 - 26, 24 - 33 | 44.32 ± 12.86; 44.02 ± 11.34 | Iran | Mild | I, II | Zufa syrup 7.5 mL Q4h + SOC for 10 days | Placebo + SOC |
| Chitre et al. (2022), (46) | RCT | M | 102 - 103 | 14 | 72 - 30, 71 - 32 | 43.0 (12.34); 41.7 (11.74) | India | Moderate | II, III | BV-4051 BID + SOC for 14 days | Placebo + SOC |
| Christian et al. (2023), (47) | RCT | S | 100 - 100 | 7 | 80 - 20, 72 - 28 | 53; 56 | India | Mild-to-severe | I-V | Siddha regimen BID + SOC for 7 days | Placebo + SOC |
| Hasanpour et al. (2022), (48) | RCT | S | 30 - 20 | 7 | 21 - 9, 13 - 7 | 48.86; 44.85 | Iran | Mild | I, II | Covexir BID + SOC for 7 days | Placebo + SOC |
| Hasheminasab et al. (2022), (49) | RCT | S | 35 - 35 | 6 | 14 - 21, 17 - 18 | 51.49 ± 11.61; 53.28 ± 13.22 | Iran | Mild-to-moderate | III, IV | Hordeum vulgare 200 mL BID + SOC for 5 days | SOC |
| Honarkar Shafie et al. (2021), (50) | RCT | M | 26 - 24 | 6 | 15 - 11, 14 - 10 | 57.46 (11.61); 57.79 (11.45) | Iran | (Hospitalized) mild-to-moderate | III, IV | 160 mg curcumin + SOC for 6 days | Placebo + SOC |
| Loc et al. (2022), (51) | RCT | S | 34 - 32 | 14 | 18 - 16, 14 - 18 | 34 (28 - 42); 35 (29 - 46) | Vietnam | Mild | I, II | Kovir capsule (TD0069) TID + SOC for 14 days | Placebo + SOC |
| Mosadegh et al. (2022), (52) | RCT | M | 35 - 35 | 14 | 19 - 16, 18 - 17 | 48.69 ± 13.00; 54.54 ± 13.92 | Iran | Critical | V-VII | NBS superfood 1.5 g TID + SOC for 14 days | Placebo + SOC |
| Patankar et al. (2022), (53) | RCT | S | 39 - 33 | 30 | 17 - 22, 20 - 13 | 47; 43 | India | Mild-to-moderate | III, IV | Each IP1 400 mg + IP2 450 mf BID for two 15 days + SOC | Placebo + SOC |
| Ratiani et al. (2022), (54) | RCT | S | 34 - 52 | 21 | 12 - 22, 24 - 28 | 49.82 (16.33); 44.73 (16.85) | Georgia | Mild | I, II | Kan Jang 90 mg QD + SOC for 14 days | Placebo + SOC |
| Said et al. (2022), (55) | RCT | S | 30 - 30 - 30 - 30 | 14 | 18 - 12, 17 - 13, 14 - 16, 21 - 9 | 29 (21 - 62); 50 (20 - 64); 44.5 (19 - 63); 26 (21 - 64) | Egypt | Mild-to-moderate | I-IV | Nigella sativa BID (arm I), vitamin D3 BID (arm II), Nigella sativa + vitamin D3 (arm III) + SOC for 14 days | SOC |
| Sankhe et al. (2022), (56) | RCT | S | 60 - 60 | 42 | 45 - 15, 44 - 16 | NA | India | Mild-to-severe | III-V | AyurCoro3 three doses on day 1 + SOC | SOC |
| Sasidharan et al. (2022), (57) | RCT | M | 58 - 58 | 10 - 15 | 48 - 10, 45 - 13 | NA | India | (Hospitalized) mild-to-moderate | III, IV | ZingiVir-H 500 mg every 3 ± 1 h + SOC for 10 - 15 days | Placebo + SOC |
| Soleiman-Meigooni et al. (2022), (58) | RCT | M | 91 - 104 | 7 | 66 - 25, 74 - 30 | 52.7 ± 19.6; 54.6 ± 15.2 | Iran | Moderate | IV | licorice 10 mL TID + SOC for 7 days | SOC |
| Taghavi et al. (2023), (59) | RCT | S | 72 - 69 | 5 | 35 - 37, 28 - 41 | 44 (41 - 54); 43 (36 - 49) | Iran | Mild-to-moderate | III, IV | Gallecina 90 mg TID + SOC for 5 days | Placebo + SOC |
| Tahmasebi et al. (2021), (60) | RCT | S | 40 - 40 - 40 | 21 | 24 - 16, 24 - 16, 24 - 16 | 54.2 ± 9.1; 54.2 ± 9.1; 52.4 ± 8.5 | Iran | Mild-to-severe | III-V | SinaCurcumin 80 mg BID + SOC for 21 days | Placebo + SOC |
| Takayama et al. (2022), (61) | RCT | M | 70 - 73 | 14 | 45 - 25, 47 - 26 | 35 (28 - 47); 37 (26 - 46) | Japan | Mild-to-moderate | I-IV | Kampo (granules of kakkonto and shosaikotokakikyosekko) TID + SOC for 14 days | SOC |
| Thakar et al. (2022), (62) | RCT | S | 41 - 39 | 14 | 26 - 15, 27 - 12 | 40 ± 12.9; 35.31 ± 11.68 | India | (Hospitalized) mild-to-moderate | III, IV | AYUSH 64 1 g TID + SOC for 14 days | SOC |
| Valizadeh et al. (2020), (63) | RCT | S | 20 - 20 - 40 | 14 | 15 - 5, 16 - 4, 30 - 10 | 53.3 ± 8.4; 51.4 ± 7.9; 49.8 ± 8.3 | Iran | Moderate-to-severe | > III | Curcumin 40 mg TID + SOC for 14 days | Placebo + SOC |
| Varnasseri et al. (2022), (64) | RCT | M | 30 - 30 | 10 | 12 - 18, 17 - 13 | 47.87 ± 14.31; 44.27 ± 11.20 | Iran | (Hospitalized) mild-to-moderate | III, IV | Amla sachet powder 2 g or Amla tea 100 cc QD + SOC for 10 days | Placebo + SOC |
| Xiong et al. (2020), (65) | RCT | S | 22 - 20 | 7 | Not reported | 57.1 ± 14; 62.4 ± 12.3 | China | Moderate-to-severe | III-V | XBD 200 mL BID + SOC for 7 days | SOC |
| Ye and G. Champs Collaborative Group (2020), (66) | RCT | S | 28 - 14 | 7 | 2 - 25, 4 - 10 | 65 (53.5 - 69); 59 (47 - 67) | China | Severe | V | CHM 200 mL BID + SOC for 7 days | SOC |
Characteristics of 50 Included Studies on the Effectiveness of Herbal Medicine Add-on Treatments for COVID-19
3.2. Effectiveness of Herbal Medicine Add-On to Standard of Care for Overall and Sign/Symptom Improvement in COVID-19
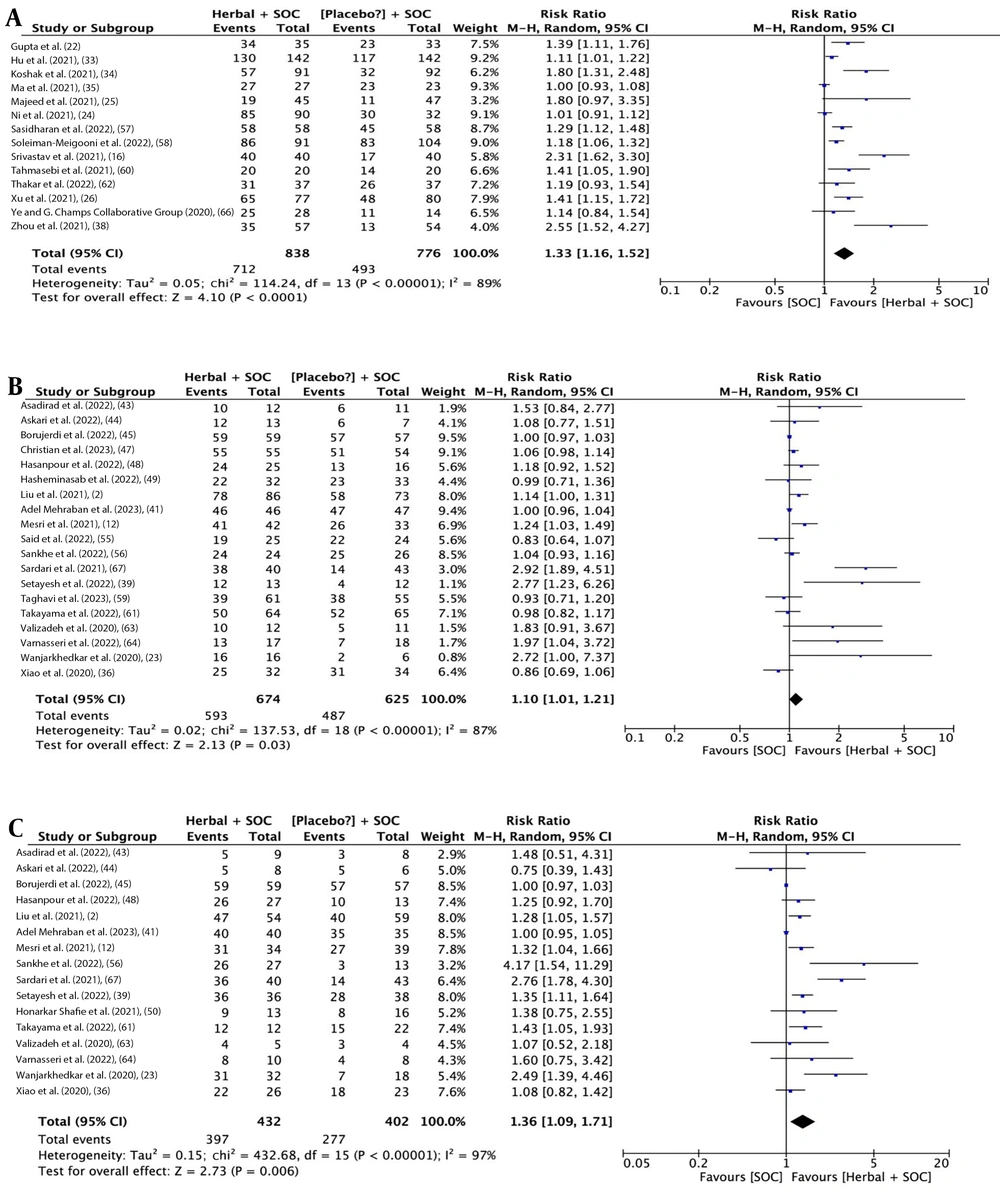
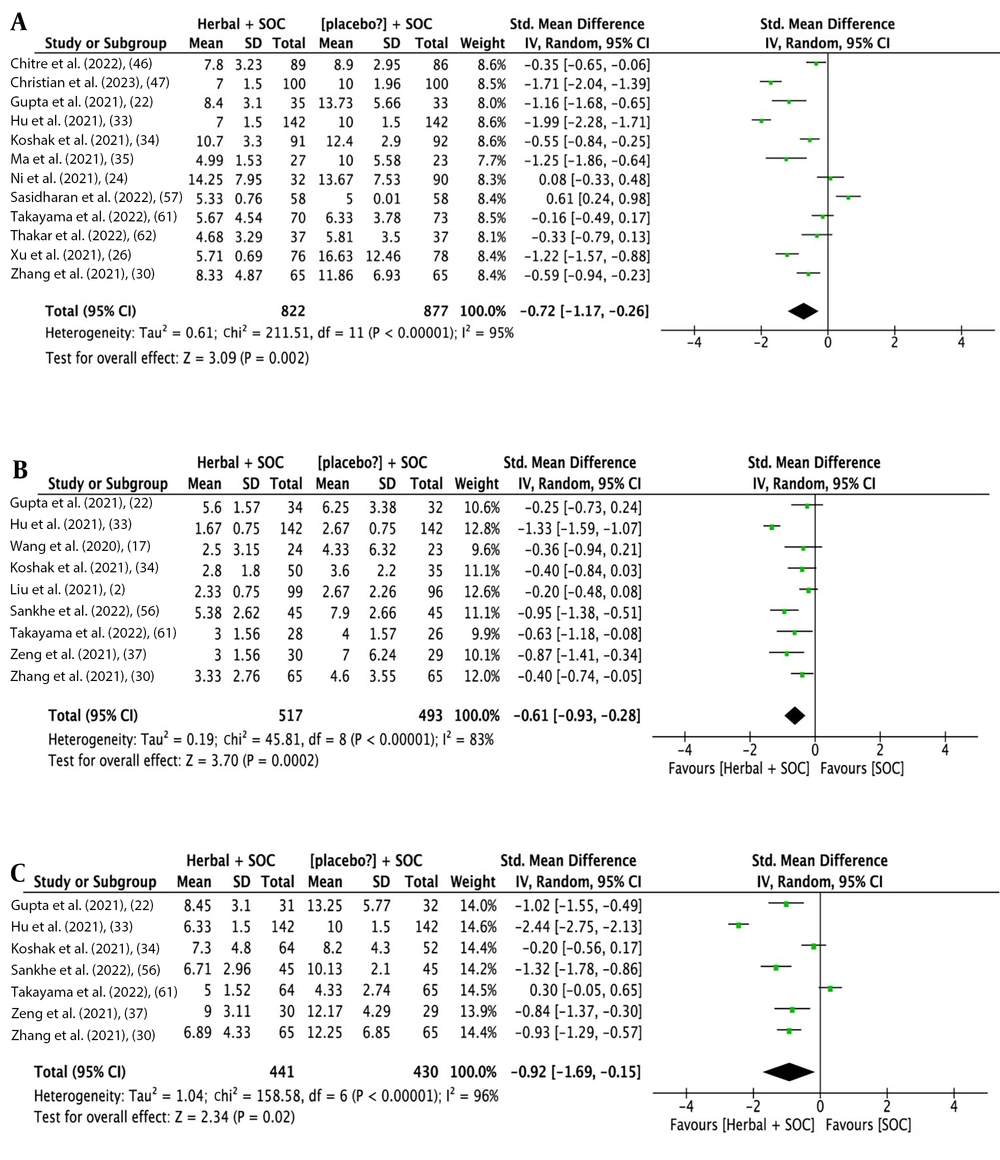
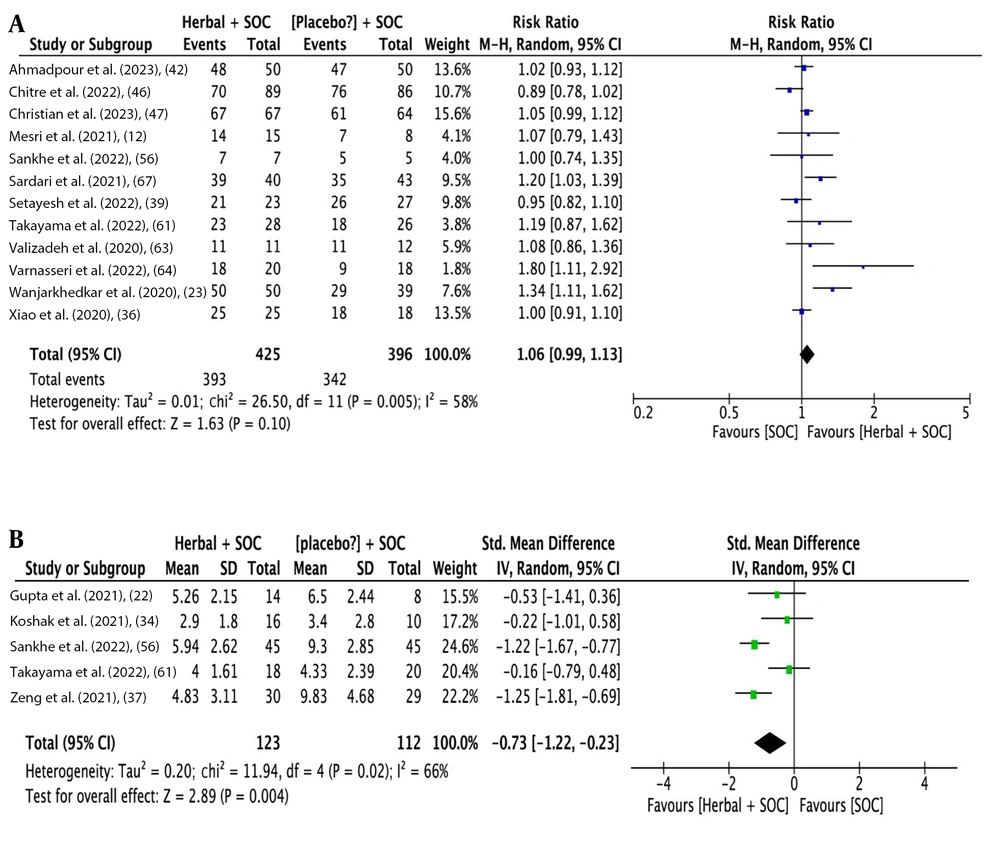
The analysis outcomes indicate that herbal medicine add-on to SOC showed a statistical difference compared to SOC alone regarding overall improvement rate [14 studies (16, 22, 24-26, 33-35, 38, 57, 58, 60, 62, 66); RR: 1.33, 95% CI: 1.16, 1.52; P < 0.0001] (Figure 3A), cough improvement rate [19 studies (2, 12, 23, 29, 36, 39, 41, 43-45, 47-49, 55, 56, 59, 61, 63, 64); RR: 1.10, 95% CI: 1.01, 1.21; P = 0.03] (Figure 3B), and dyspnea improvement rate [16 studies (2, 12, 23, 29, 36, 39, 41, 43-45, 48, 50, 56, 61, 63, 64); RR: 1.36, 95% CI: 1.09, 1.71; P = 0.006] (Figure 3C). Additionally, there was a statistical difference in the time needed for overall recovery [12 studies (22, 24, 26, 30, 33-35, 46, 47, 57, 61, 62); SMD: -0.72 days, 95% CI: -1.17, -0.26; P = 0.002] (Figure 4A), time needed for recovery from fever [9 studies (2, 17, 22, 30, 33, 34, 37, 56, 61); SMD: -0.61 days, 95% CI: -0.93, -0.87; P < 0.0001] (Figure 4B), time needed for recovery from cough [7 studies (22, 30, 33, 34, 37, 56, 61); SMD: -0.92 days, 95% CI: -1.69, -0.15; P = 0.02] (Figure 4C), and time needed for recovery from dyspnea [5 studies (22, 34, 37, 56, 61); SMD: -0.73 days, 95% CI: -1.12, -0.23; P = 0.004] (Figure 5A). No statistical difference was found for fever improvement rate [12 studies (12, 23, 36, 39, 42, 46, 47, 56, 61, 63, 64, 67); RR: 1.06, 95% CI: 0.99, 1.13; P = 0.10] (Figure 5B).
Figures 3 to 5 illustrate the forest plots for (A) overall improvement rate, (B) cough improvement rate, (C) dyspnea improvement rate, (A) time needed for overall recovery, (B) recovery from fever, (C) recovery from cough, (A) fever improvement rate, and (B) time needed for recovery from dyspnea by herbal medicine add-on to SOC versus SOC for COVID-19.
High heterogeneity was observed in all the aforementioned meta-analyses. After removing outlying studies, heterogeneity improved across all outcomes to varying degrees, and the recalculated summary effect sizes confirmed all the full-set meta-analyses, except for the lack of beneficial effects of herbal medicine add-on therapies in terms of cough improvement rate (RR: 1.03, 95% CI: 0.98, 1.08; P = 0.22) (Table 2).
| Outcomes | Heterogeneity | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|
| tau2 | χ2 | P-Value | I2 (%) | RR, SMD | 95% CI | Z | P-Value | |
| Overall improvement (referred forest plot: Figures 3A and 5A) | ||||||||
| Rate, Koshak et al. (34); Ni et al.(24); Ma et al. (35); Srivastava et al. (16); Zhou et al. (38) a | 0.00 | 11.58 | 0.17 | 31 | 1.23 | 1.15, 1.33 | 5.59 | < 0.00001 |
| Time needed for recovery; Xu et al. (26); Gupta et al. (22); Christian et al. (47); Hu et al. (33); Srivastava et al. (16) | 0.06 | 17.02 | 0.01 | 65 | -0.41 | -0.65, -1.17 | 3.38 | 0.0007 |
| Fever improvement (referred forest plot: Figures 4B and 5A) | ||||||||
| Rate; Varnasseri et al. (64) | 0.00 | 19.67 | 0.03 | 49 | 1.04 | 0.98, 1.11 | 1.43 | 0.15 |
| Time needed for recovery; Hu et al. (33) | 0.03 | 11.66 | 0.11 | 40 | -0.48 | -0.68, -0.28 | 4.70 | < 0.00001 |
| Cough improvement (referred forest plot: Figures 3B and 4c) | ||||||||
| Rate; Serdari et al. (67); Setayesh et al. (39); Varnasseri et al. (64); Wanjarkhedkar et al. (23) | 0.00 | 26.66 | 0.02 | 47 | 1.03 | 0.98, 1.08 | 1.23 | 0.22 |
| Time needed for recovery; Koshak et al. (34); Hu et al. (33); Takayama et al. (61) | 0.00 | 2.35 | 0.50 | 0 | -1.03 | -1.25, -0.80 | 8.87 | < 0.00001 |
| Dyspnea improvement (referred forest plot: Figures 3C and 5B) | ||||||||
| Rate; Sardari et al. (67); Borujerdi et al. (45); Adel Mehraban et al. (41) | 0.02 | 18.17 | 0.11 | 34 | 1.33 | 1.17, 1.51 | 4.30 | 0.0005 |
| Time needed for recovery; Takayama et al. (61) | 0.12 | 6.46 | 0.09 | 54 | -0.90 | -1.37, -0.43 | 3.74 | 0.0002 |
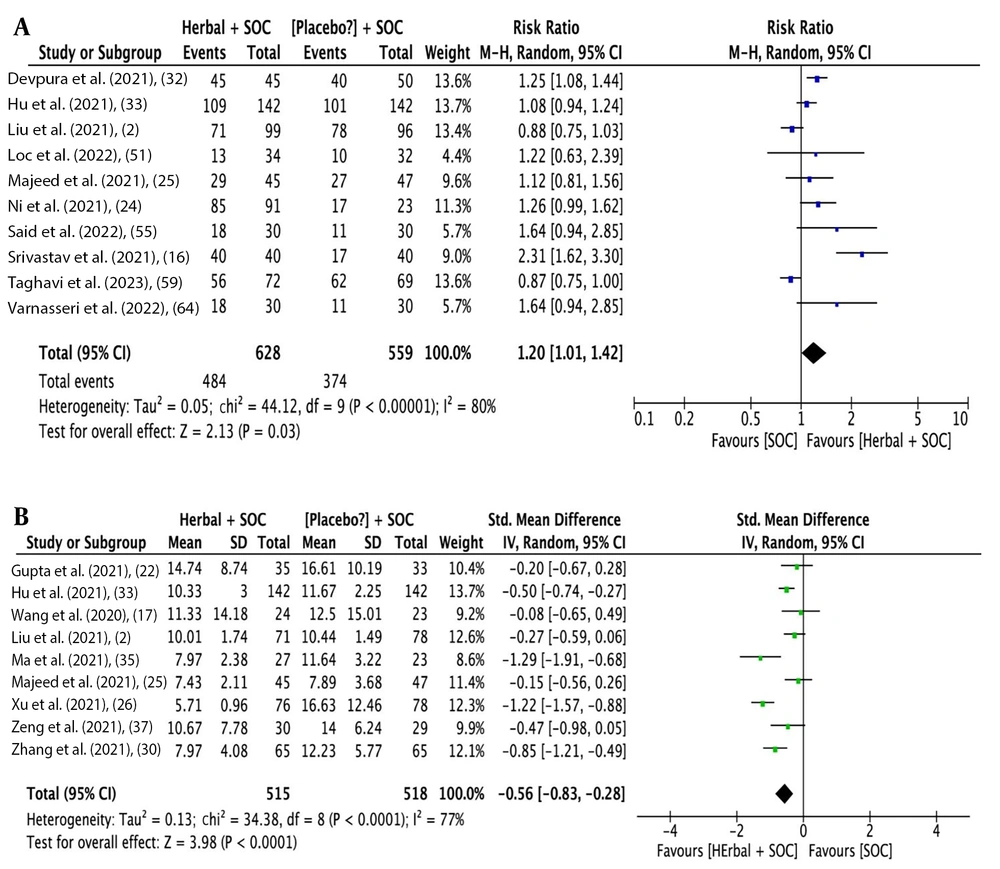
| RT-PCR conversion into negative (referred forest plot: Figure 6A and B) | ||||||||
| Rate; Liu et al. (2); Srivastava et al. (16); Taghavi et al. (59) | 0.00 | 5.23 | 0.52 | 0 | 1.19 | 1.09, 1.29 | 3.91 | < 0.0001 |
| Time needed for conversion; Xu et al. (26); Ma et al. (35) | 0.03 | 10.71 | 0.10 | 44 | -0.39 | -0.59, -0.20 | 3.93 | < 0.0001 |
| Deterioration rate b (referred forest plot: Appendix 4- part Ain Supplementary File) | - | - | - | - | - | - | - | - |
| Hospital LoS c (referred forest plot: Appendix 4- part B in Supplementary File) | - | - | - | - | - | - | - | - |
Meta-analysis Results of Sensitive Analysis for Outcomes in Comparing Herbal Medicine Add-on to Standard of Care Either Standard of Care for COVID-19
3.3. Effectiveness of Herbal Medicine Add-on to Standard of Care for RT-PCR Conversion to Negative for COVID-19
The analysis outcomes indicate that herbal medicine add-on to SOC showed a statistical difference compared to SOC alone for RT-PCR conversion rate [10 studies (2, 16, 24, 25, 32, 33, 51, 55, 59, 64); RR: 1.20, 95% CI: 1.01, 1.42; P = 0.03] (Figure 6A) and time needed for RT-PCR conversion [9 studies (2, 17, 22, 25, 26), (30, 33, 35, 37); SMD: -0.56 days, 95% CI: -0.83, -0.28; P < 0.0001] (Figure 6B). Considering the presence of high heterogeneity, sensitivity analysis improved heterogeneity and showed statistically favorable results for herbal medicine add-on to SOC (Table 2).
3.4. Effectiveness of Herbal Medicine Add-On to Standard of Care for Deterioration Rate for COVID-19
The analysis outcomes indicate that herbal medicine add-on to SOC showed a statistically lower worsening rate compared to SOC alone (2, 12, 17, 19, 22, 23, 28 ,30, 31, 33, 34, 36, 37, 42, 27, 43, 46-53, 57-64, 65, 66); RR: 0.47, 95% CI: 0.38, 0.58; P < 0.00001) (Appendix 4, Part A in Supplementary File). No heterogeneity was observed.
3.5. Effectiveness of Herbal Medicine Add-on to Standard of Care for Hospital Length of Stay for COVID-19
The analysis outcomes indicate that patients who received herbal medicine add-on to SOC had shorter hospital stays than those who did not receive herbal medicine add-on (16, 22, 25-28, 35, 37, 39, 40, 47, 49, 51, 52, 58, 59); SMD: -0.60 days, 95% CI: -1.04, -0.16; P = 0.007) (Appendix 4, Part B in Supplementary File). Although high heterogeneity was observed, it was not improved by sensitivity analysis (Table 2).
The application of traditional medicine in managing infectious diseases, especially respiratory ones, is widely discussed (68-71). Experiences with herbal medications suggest a potential role in COVID-19 management (72, 73). Importantly, most studies were not placebo-controlled and exhibited heterogeneities in SOC regimen, inclusion/exclusion criteria, adverse events, and definitions (worsening, improvement, etc.). Our study highlights degrees of improvement in some outcomes using herbal drugs in COVID-19 patients.
3.6. Hospital Length of Stay
Our results indicate an approximate reduction of 0.16 to 1.04 days in hospital LoS by adding herbal therapies to the SOC, despite noticeable heterogeneity and varying definitions. A study reviewing the effect of integrated traditional Chinese medicine and Western medicine also reports a shorter hospital LoS in integrated regimens compared to those receiving Western medicine regimens alone (74).
3.7. PCR Conversion Rate and Time
Using herbal medicine as an add-on to the SOC regimen is associated with an increased rate of PCR conversion to negative (RR = 1.01 to 1.42) and decreased time needed for conversion (0.28 to 0.83 days). However, a systematic review and meta-analysis by Du et al. on the effect of Honeysuckle in COVID-19 patients reveals no statistically significant difference (75).
3.8. Disease Deterioration
Using herbal medicine in combination with SOC is associated with significantly less disease progression (RR = 0.47). This finding is supported by a study reporting a lower rate of conversion to severe disease after adding herbal drugs to the therapeutic regimen (75). Based on a retrospective study by Feng et al. (76), the probability of developing ARDS and cardiac injury was lower in the treatment group, as was the likelihood of requiring mechanical ventilation. Integrated medicine regimens can decrease the rate of progression to severe illness and improve the cure rate as well (74).
3.9. General Improvement
Our findings show that using herbal medicine can increase the rate of general sign/symptom improvement (RR = 1.16 to 1.52) with a decrease in the time needed for resolution (SMD = -0.26 to -1.17 days). A study reports considerable improvement in symptom scores of fatigue but not in the fatigue reduction rate (75). Integrating medicine is associated with some general symptom improvement, increased disappearance rate, and decreased disappearance duration (74). The rate of general symptoms disappearing was reported to be higher in those receiving traditional Chinese medicine (77).
3.10. Fever Improvement
The effect of herbal medicine on the rate of fever resolution was not significant (RR = 0.99 to 1.13); however, the time to become fever-free is decreased (SMD = -0.87 to -0.93 days). An improvement in fever scores was found in a study, contrasting with the fever reduction rate (75). The rate of fever fading and the duration of fever were higher and lower, respectively, in the integrated group (74). Another study reported an increased disappearance rate and decreased duration of fever compared to controls (77).
3.11. Cough Improvement
The rate of becoming cough-free was lower in those receiving SOC (RR = 1.01 to 1.21); additionally, using herbal treatments is associated with a shorter time to become cough-free (SMD = -0.15 to -1.69 days). Combination therapy improved both cough reduction rate and cough scores (75). Zeng et al. support the idea that the disappearance rate of cough is higher in those receiving Chinese medicine (77).
3.12. Dyspnea Improvement
Patients receiving SOC had higher rates of becoming dyspnea-free (RR = 1.09 to 1.71), but the resolution time differed between the two groups (SMD = -0.23 to -1.12 days). The disappearance rate of dyspnea (difficulty breathing) is reported to be higher in another study (77). In line with these findings, an observational study in South Korea reports improvement in all COVID-19 symptoms, whether general or more specific ones (cough, dyspnea, fever, etc.) (78).
4. Conclusions
The results of this study will aid in reaching a consensus regarding the application of such alternatives. Additionally, it can guide governments and health organizations in establishing efficient and practical policies to enhance the quality of care for patients with COVID-19 and improve prognosis. Several significant limitations affect the interpretation of our meta-analysis. As mentioned, most studies were not placebo-controlled trials. The SOC for COVID-19 patients has been constantly evolving in terms of time, protocol, and country. The heterogeneous case selection with variable inclusion and exclusion criteria (Appendix 3 in Supplementary File) is another debatable issue. Studies with a high number of adverse effects might include abnormal lab results as adverse events. Additionally, there was varying sensitivity for symptoms, ranging from 0% to 85% for experimental and 0% to 89% for control groups.
Moreover, the heterogeneity in defining concepts such as clinical improvement and worsening (need for hospitalization, O2 therapy, ARDS, etc.), and the different herbal drugs used in each study, which enabled us to perform sensitivity analysis, are other limitations of our study. High heterogeneity was a major issue, potentially stemming from various factors, including differences in doses used in experiments, treatment durations, and treatment-assessment endpoint periods across studies. Additionally, the inclusion of a diverse range of herbal remedies compounded the variability because different herbs and formulations may have varying mechanisms of action and efficacy. Grouping them together in a meta-analysis might obscure the effects of individual herbs; however, due to the limited number of RCTs available for each individual herb, subgroup analyses could not be performed, which may have further masked specific effects.
While meta-regression is a useful tool for exploring associations between study-level characteristics and outcomes, it may not be the most appropriate method in this case due to the specific limitations of our dataset. The inclusion of a diverse range of herbal remedies introduces substantial variability, complicating the interpretation of meta-regression results. Additionally, meta-regression typically requires a larger number of studies to yield reliable and meaningful insights, and given the limited number of RCTs available for each individual herb, the power of any analysis may be insufficient.
The quality of the included studies was another major limitation, potentially decreasing the reliability of the results and the overall validity of the meta-analysis. Additionally, our meta-analysis was limited by language and publication bias, as we included only RCTs published in English, although this issue was less pronounced according to the visual inspection of the corresponding funnel plots (Appendix 5 in Supplementary File). Overall, the limitations observed in the included RCTs necessitate cautious interpretation of the results. Notably, possible contributing factors for heterogeneity may become apparent when the results of ongoing trials become available.
In summary, although our meta-analysis suggests that the application of traditional herbal medicine add-on treatments in COVID-19 patients might hold potential for improvements in some outcomes, the evidence remains inconclusive, primarily due to unexplained high levels of between-study heterogeneity and methodological limitations, making it challenging to reach a definite conclusion. Further well-designed controlled placebo trials with rigorous methodologies are essential to clarify the true impact of herbal drugs in such diseases. Future research should aim to address these methodological gaps and provide more robust data to guide clinical practice. In the interim, herbal remedies may be considered as complementary options for these patients.