1. Background
The presence of the membrane is a decisive and essential element in maintaining the vital functions of any living cell and plays an important role in maintaining the homeostatic environment and protecting the inner cell organelles (1). The molecular structure of the cell membrane consists of a variety of interconnected lipids and is associated with various protein components (2). The main glycerophospholipid that forms the membrane of eukaryotic cells is phosphatidylcholine (PC), followed by other important glycerophospholipids such as phosphatidylethanolamine, phosphatidylserine, phosphatidylinositol, and phosphatidic acid (3). The polar head of the lipid groups has hydrophilic properties, facing the cytosol, while the hydrocarbon tails have hydrophobic properties, forming the inner space of the bilayer membrane with a thickness of approximately 5 nm (4).
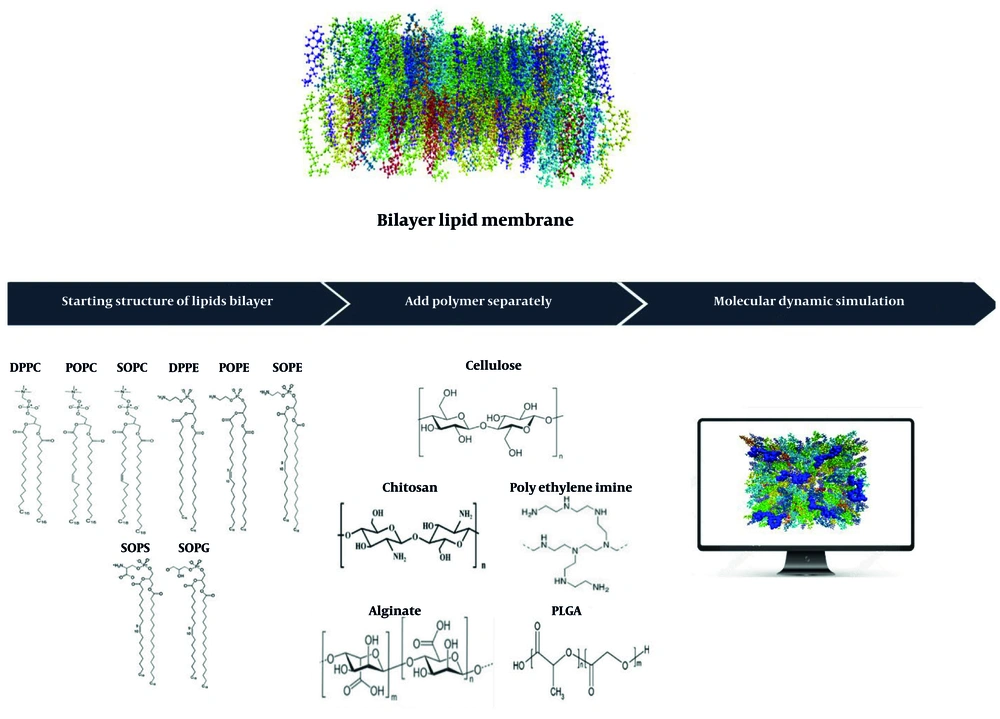
The selection of the appropriate carrier is a crucial factor in the design of new systems for drug delivery (5-7). This process in the body requires various types of polymers with different physicochemical properties such as molecular weight and charge, among others. These differences affect their interaction with lipid membranes (8). Important polymers widely utilized in pharmaceutical formulations include chitosan, polyethyleneimine (PEI), alginate, polylactic-co-glycolic acid (PLGA), and cellulose (9).
The molecular dynamics (MD) simulation method is a useful time-dependent scientific tool to investigate the structural and dynamic information of various and complex systems such as biological membranes and to observe functional processes such as ligand binding, protein folding, and membrane transport. It can provide predictions of biological responses to modifications including mutation, phosphorylation, protonation, or interaction with other molecules/materials (10). By using the results obtained from this method, it is possible to understand molecular behavior at atomic details in order to predict the interaction of different polymer carriers with lipid membranes (11).
The development of effective drug delivery systems is a critical challenge in the pharmaceutical industry. One approach to achieving this goal is to design polymer-based carriers that can interact with biological membranes and facilitate the delivery of therapeutic agents. However, the interactions between polymers and biological membranes are complex and not yet fully understood. Recent studies have shown that polymers can interact with lipid bilayers through a variety of mechanisms, including electrostatic and hydrophobic interactions (12-14), leading to changes in membrane structure and function (15, 16). However, the effects of polymer properties on membrane disruption are not yet well understood.
Types of important natural and synthetic biodegradable polymers widely utilized in pharmaceutical formulations include cellulose, chitosan, PEI, alginate, and PLGA were used in this study (9).
2. Objectives
Our study aims to address this knowledge gap by investigating the interactions between a range of biological and synthetic polymers and a lipid bilayer membrane using MD simulations. This knowledge will be essential for the design of effective and safe drug delivery systems.
3. Methods
The GROMACS molecular package version 2021, with the CHARMM36 all-atom force field, was used to conduct simulations of various polymers interacting with a lipid bilayer membrane (17). The coordinate and topological information related to the starting structures of the lipid bilayer and polymers were obtained from the CHARMM-GUI server (https://www.charmm-gui.org/).
The lipid content and their constituent percentages were prepared to mimic those of the human cell membrane, including cholesterol, dipalmitoylphosphatidylcholine (DPPC), 1-palmitoyl-2-oleoylphosphatidylcholine (POPC), 1-stearoyl-2-oleoylphosphatidylcholine (SOPC), dipalmitoylphosphatidylethanolamine (DPPE), 1-palmitoyl-2-oleoylphosphatidylethanolamine (POPE), 1-stearoyl-2-oleoyl-phosphatidylethanolamine (SOPE), stearoyloleoylphosphatidylserine (SOPS), and 1-stearoyl-2-oleoyl phosphatidylglycerol (SOPG) (Table 1). The percentage and number of each lipid in the model membrane were selected based on a literature review (18-20).
| Lipids | Top Layer | Bottom Layer |
|---|---|---|
| Cholesterol | 30 | 30 |
| POPE | 6 | 15 |
| DPPE | 3 | 10 |
| SOPE | 6 | 15 |
| POPC | 20 | 8 |
| DPPC | 15 | 5 |
| SOPC | 20 | 7 |
| SOPS | 0 | 9 |
| SOPG | 0 | 1 |
Percentage of Lipids Forming the Membrane
For polymer selection, two positively charged polymers, chitosan and PEI, with high and low molecular weights, two negatively charged polymers, alginate and PLGA, with high and low molecular weights, and cellulose as a neutral polymer were used to investigate the interaction of polymers with the bilayer membrane regarding their charges and molecular weights of monomers (Figure 1).
A certain number of each polymer was randomly placed on the outer layer of the membrane so that the final systems were equal, based on their molecular weight. The TIP3P water model (21) was used for solvation of the system, and to completely neutralize the charge, an appropriate number of counter ions (Na+ and Cl-) were added to the simulation boxes. Thus, Na+ ions were added to the boxes containing negatively charged polymers, and Cl- ions were added to the boxes containing positively charged polymers. The water model is recommended by the software developer as a more suitable model for working with the CHARMM force field.
Energy minimization of the systems was performed using the steepest descent algorithm until the maximum force on the atoms reached a final value of < 10 kJ/mol.nm (22). Periodic boundary conditions (PBC) were applied in all X, Y, and Z directions to eliminate energy drifts resulting from edge effects in the small simulation boxes. To simulate real body conditions, the temperature and pressure were adjusted to 310 K and 1 bar using NVT and NPT ensembles for 200 ps. In this regard, the temperature and pressure were equilibrated using the Hoover thermostat, Nose thermostat and Parrinello and Rahman barostat, respectively. Both methods are precise algorithms for coupling the conditions of molecular simulation (23-25). The linear constraint solver (LINCS) algorithm was employed to keep all bonds around their equilibrium value (26). Short-range van der Waals and Coulombic interactions were calculated for all particles within a cut-off distance of 1 nm. The particle-mesh Ewald (PME) method (27) was also applied for the calculation of long-range electrostatic interactions. This method helps manage the atomic forces between particles outside the central box when PBC is applied in the simulation.
Eventually, all simulations were conducted for 100 nanoseconds under the default leap-frog algorithm in the GROMACS package. The output structure of the simulated systems was prepared with the visual molecular dynamics (VMD) package (28). The interaction energies were calculated using the MM/PBSA method (29), and the GridMAT-MD tool (30) was used for thickness parameter measurements.
4. Results and Discussion
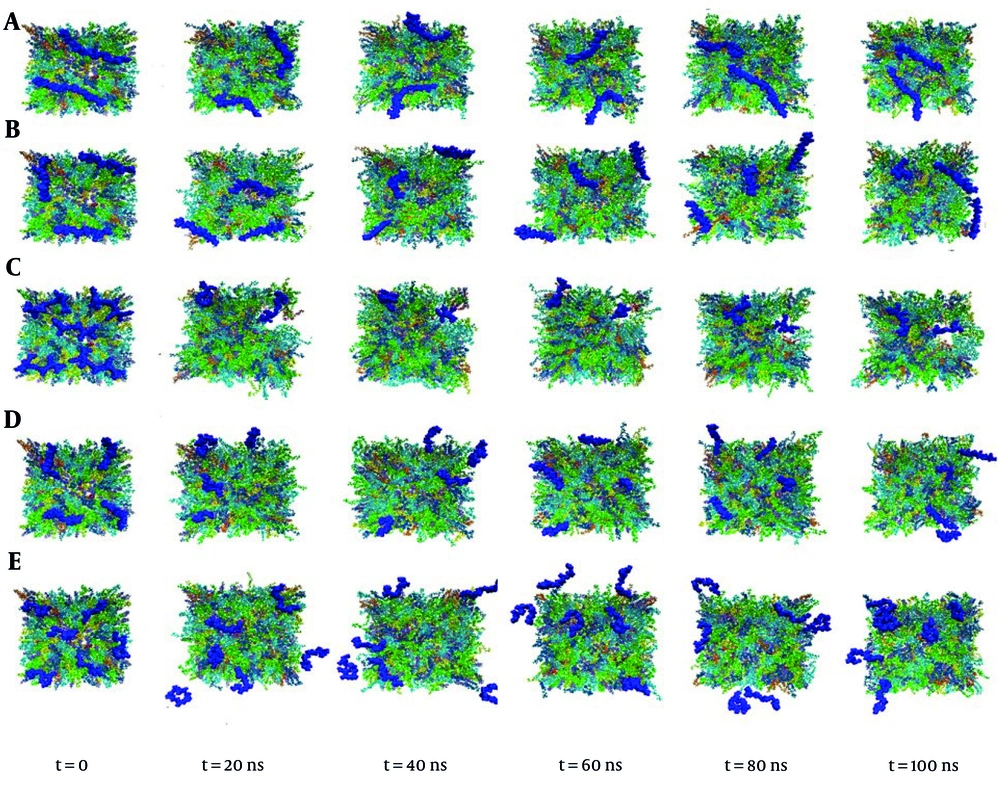
The interaction of different pharmaceutical polymers used as carriers in drug delivery systems was investigated with a biological cell membrane model using the MD simulation method. This study aimed to investigate the interaction of various polymers with different molecular weights and charges. As a positively charged polymer, PEI interacted significantly with the lipid membrane compared to other polymers and caused damage in a region of the membrane (Figure 2). The low molecular weight of this polymer allows it to pass through membrane lipids, leading to a significant effect on the performance and behavior of the membrane (Figure 2C). In contrast, PLGA showed less interaction with the lipid bilayer compared to other polymers, possibly due to its negative charge and fewer hydrogen bonding donor-acceptor groups in comparison with others.
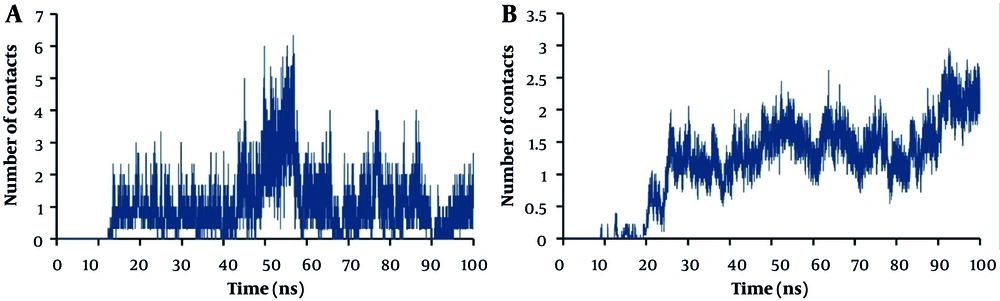
4.1. Number of Contacts
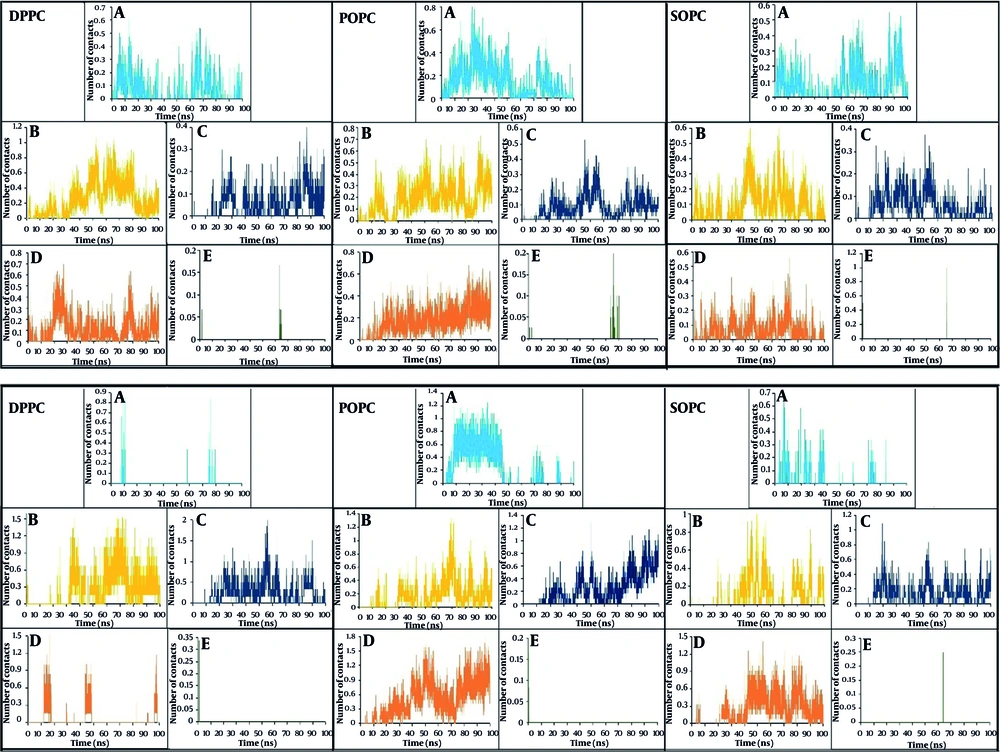
The number of contacts between specific groups of atoms is investigated during MD simulations to ensure stable interactions. The results of the normalized number of contacts per number of molecules between polymers and lipids of the bilayer membrane are shown in Figure 3. The results indicate that, except for PLGA, all other polymers interact with almost all lipids and form a close complex with the membrane. This can be attributed to the negatively charged molecules and lack of sufficient hydrogen bonding donors in the structure of PLGA. In general, choline-containing lipids show higher collisions with polymers than ethanolamine-containing lipids.
The interaction of PEI with lipids, especially anionic lipids like SOPG and SOPS, on the inner side of membranes increases significantly after passing through the membrane. This interaction is driven by electrostatic forces between the positively charged PEI and the negatively charged phosphate groups of SOPG and SOPS anionic lipids (Figure 4). The limited interaction of PLGA with the lipid bilayer is likely due to its negatively charged molecules and lack of hydrogen bonding donors. This suggests that PLGA may not be as effective at disrupting the membrane or delivering drugs across the membrane.
4.2. Interaction Energy
The binding free energy between the polymer and lipids of the bilayer membrane was calculated using the MM-PBSA method in the g_mmpbsa software. The variation in binding energy among different polymers can indicate differences in their interactions with lipids (31). Based on the results reported in Table 2, there is a strong affinity between all of the polymers and PC lipids compared to phosphatidylethanolamine (PE) lipids, which aligns with the results obtained from analyzing the number of contacts. The only exception is observed in the system containing PLGA, where, despite the lack of close contacts, the low interaction energies compared to other polymers are notable. Considering the negative charge of PLGA and the presence of a positive center on the nitrogen atom of the choline molecule, such strong electrostatic interaction energy is acceptable. Among the polymers, cellulose and PEI have the lowest and highest binding energy, respectively. These results confirm the importance of electrostatic interactions between the molecules, which may lead to different effects of polymers on biological systems.
| Polymer Lipid | Cellulose | Chitosan | PEI | Alginate | PLGA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vdW | Elec | Total | vdW | Elec | Total | vdW | Elec | Total | vdW | Elec | Total | vdW | Elec | Total | |
| DPPC | -6 | -9 | -16 | -38 | -154 | -192 | -18.6 | -1299 | -1317 | -27 | -418 | -446 | -0.12 | -78 | -79 |
| DPPE | -0.2 | -1 | -1 | -10 | -99 | -109 | -7.06 | -943 | -950 | 0.1 | -78 | -77 | -0.02 | -0.7 | -0.8 |
| POPC | -16 | -32 | -48 | -39 | -405 | -445 | -38 | -2498 | -2537 | -80 | -744 | -824 | -0.14 | -89 | -89 |
| POPE | -1 | -9 | -11 | -19 | -191 | -210 | -17 | -2520 | -2538 | -24 | -1102 | -1126 | -0.05 | 3.5 | 3.5 |
| SOPC | -32 | -67 | -99 | -22 | -42 | -64 | -17 | -1495 | -1512 | -16 | -521 | -538 | -0.34 | -101 | -102 |
| SOPE | -0.35 | -0.47 | -0.8 | -0.35 | -0.47 | -0.82 | -20 | -1333 | -1354 | -23 | -431 | -454 | -0.06 | -2.1 | -2.2 |
| SOPG | - | - | - | - | - | - | -11 | -717 | -728 | - | - | - | - | - | - |
| SOPS | - | - | - | - | - | - | -27 | -5400 | -5428 | - | - | - | - | - | - |
Interaction Energies between Lipids and Polymers Obtained by mmpbsa
4.3. Membrane Thickness
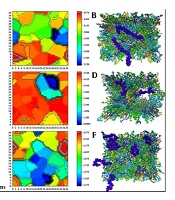
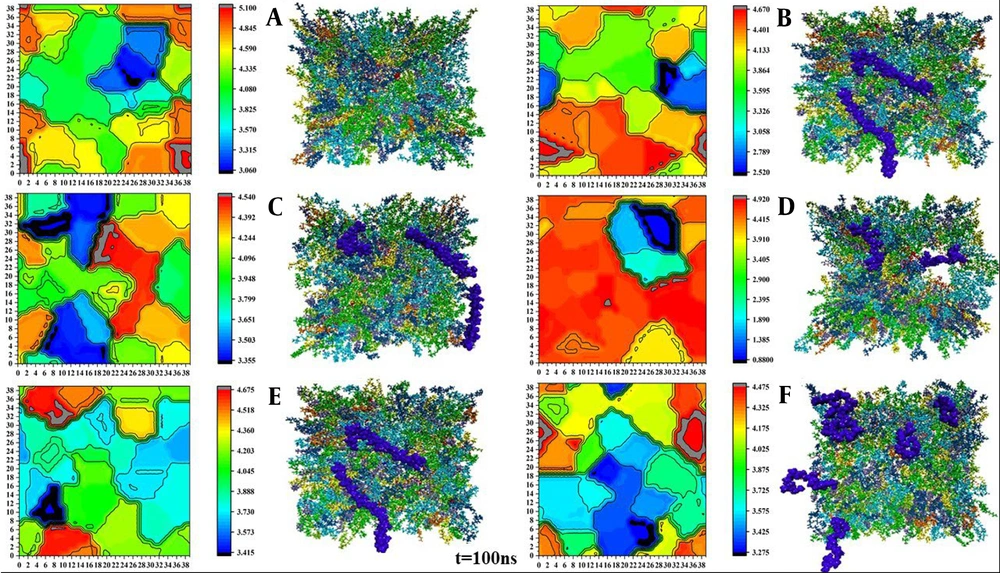
The overall membrane thickness, consisting of a bilayer of lipids, is approximately 4 nm. An increase in membrane thickness following changes inside or around the membrane can have various effects on biomembrane stability and cell function (32). The thickness of the final structure related to the lipid membrane with polymer in a 40 × 40 matrix was calculated using the GridMAT-MD program. Subsequently, the obtained results were visualized using OriginPro (Figure 5).
Among the different polymers, PEI exhibited the most significant effect on the membrane by disrupting the lipid bilayer and causing a significant increase in its thickness. The penetration of PEI into the membrane resulted in localized compression in the membrane thickness. In addition, these changes can significantly affect other membrane properties, such as lipid order. After adding this polymer, the order parameter changed significantly compared to other polymers. Conversely, the blue areas depicted in the figure are more prominent and are associated with chitosan, alginate, and PLGA polymers, indicating that these polymers have caused membrane compression in those specific regions. Cellulose and alginate showed a more consistent membrane thickness pattern with the free membrane, indicating the lower effects of these polymers on the bilayer.
4.4. Order Parameter
The order parameter is an essential tool used to analyze the orientation and measure the alignment of lipid chains in the bilayer membrane. The alignment and orientation of lipid chains in the membrane are considered based on the angle between the z-axis of the simulation box and an atomic vector. For each atom Cn, the vector is derived from atoms Cn-1 to Cn+1, with the final order parameter ranging from -0.5 to 1. This range indicates that two vectors are perpendicular (value of -0.5) or parallel to each other (value of 1) (33).
The results related to the order parameter for each of the free and polymer-interacted membrane lipids are represented in Figure 6. In these graphs, there is a high disorder in the head groups of both chains related to all lipids. Specifically, strong fluctuations and bumps are observed in the sn-1 chain of all lipids except DPPC and DPPE, attributed to the presence of double bonds and the creation of an unsaturated state in the oleoyl chain of these lipids. These characteristics correspond to a specific state within the graph. On the other hand, the order value of the tails decreases towards the end of the acyl chains, indicating that the tails become more disordered and flexible towards the center of the bilayer.
According to the graphs, PEI significantly influenced the order of all lipids compared to other polymers. Ignoring the PEI-containing system, the SOPG lipid order in the membrane with all of the polymers except cellulose has increased and become more rigid compared to the free membrane. The opposition of this result with that obtained for SOPC suggests that these lipids may be in close contact with each other. Also, as can be seen from the results, except for PEI, none of the polymers have shown a severe undesirable effect on the lipid order parameter, indicating their safety for use in pharmaceutical formulations.
4.5. Lateral Diffusion
Lateral diffusion is a fundamental property of biological membrane lipids that refers to the lateral movement of lipids and proteins within each leaflet of the bilayer (34). The analysis of choice for investigating molecular diffusions in MD simulations is mean squared displacement (MSD). Figure 7 provides a representation of the influence of polymers on the lateral diffusion of lipids in comparison with the free membrane over the MD simulations.
Lateral diffusion of lipids in bilayers for a, dipalmitoylphosphatidylcholine (DPPC); b, dipalmitoylphosphatidylethanolamine (DPPE); c, 1-palmitoyl-2-oleoylphosphatidylcholine (POPC); d, 1-palmitoyl-2-oleoylphosphatidylethanolamine (POPE); e, 1-stearoyl-2-oleoylphosphatidylcholine (SOPC); f, 1-stearoyl-2-oleoyl-phosphatidylethanolamine (SOPE); g, 1-stearoyl-2-oleoyl phosphatidylglycerol (SOPG); and h, stearoyloleoylphosphatidylserine (SOPS) in interaction between polymers. [A, Free membrane; B, cellulose; C, chitosan; D, polyethyleneimine (PEI); E, alginate; and F, polylactic-co-glycolic acid (PLGA)].
The highest value of MSD is observed for POPC lipids with chitosan, and the PEI polymer also has a greater effect on the displacement of POPC and SOPC lipids compared to other lipids. On the other hand, the lowest displacement is related to DPPE and two lipids, POPC and SOPS, in interaction with PLGA and cellulose, respectively. Overall, the results in Figure 5 confirm that the least deviation in MSD diagrams for all lipids can be seen in the system integrated with cellulose and PLGA. In contrast, the polymers PEI and chitosan cause the highest MSD deviations among the different membrane lipids.
Table 3 demonstrates that, when compared to the free membrane, only the DPPC lipid displays an increase in the lateral diffusion coefficient with all polymers, with a more pronounced effect reported in interaction with cellulose and chitosan polymers. Additionally, the diffusion coefficient of all lipids in interaction with PEI has been found to increase; this faster diffusion can increase membrane permeability. The highest value is observed for POPC (D = 0.0086).
| Polymer Lipid | D (cm2/s) | |||||||
|---|---|---|---|---|---|---|---|---|
| Free Membrane | Cellulose | Chitosan | PEI | Alginate | PLGA | Mean | STDEV | |
| DPPC | 0.005 | 0.0072 | 0.0072 | 0.0067 | 0.0057 | 0.0062 | 0.0066 | 0.0006 |
| DPPE | 0.0065 | 0.007 | 0.0052 | 0.0072 | 0.0071 | 0.0042 | 0.0061 | 0.0014 |
| POPC | 0.0069 | 0.0053 | 0.0081 | 0.0086 | 0.0063 | 0.0066 | 0.0069 | 0.0013 |
| POPE | 0.0045 | 0.0067 | 0.0075 | 0.0069 | 0.0042 | 0.0055 | 0.0061 | 0.0013 |
| SOPC | 0.0066 | 0.0059 | 0.0065 | 0.0084 | 0.0064 | 0.0068 | 0.0068 | 0.0009 |
| SOPE | 0.0053 | 0.0067 | 0.0054 | 0.005 | 0.0045 | 0.0073 | 0.0058 | 0.0011 |
| SOPG | 0.0035 | 0.0056 | 0.0025 | 0.0038 | 0.0033 | 0.0062 | 0.0042 | 0.0015 |
| SOPS | 0.0055 | 0.0052 | 0.0041 | 0.0055 | 0.0037 | 0.0051 | 0.0047 | 0.0007 |
| Mean | 0.0054 | 0.0062 | 0.0058 | 0.0065 | 0.0051 | 0.0059 | - | - |
| STDEV | 0.0011 | 0.0007 | 0.0018 | 0.0016 | 0.0014 | 0.001 | - | - |
Diffusion Coefficients of Lipids in Interaction with Polymers
5. Conclusions
In conclusion, this study provides a comprehensive understanding of the interactions between various polymers — cellulose, chitosan, PEI, alginate, and PLGA — and a lipid bilayer membrane using MD simulations. Our results show that the polymers interact with the lipid bilayers through a combination of electrostatic and hydrogen bonding forces, with PEI exhibiting the most significant effect on membrane disruption. The study also highlights the importance of understanding the binding free energy between polymers and lipids, with PEI having the highest binding energy.
The order parameter analysis reveals that PEI significantly influences the order of all lipids, while cellulose and alginate cause lower order and higher flexibility in the acyl chain of the lipids. The lateral diffusion analysis shows that PEI has a significant effect on the lateral diffusion of POPC and SOPC lipids, indicating a higher lateral diffusion coefficient.
Our findings have important implications for the design of effective and safe drug delivery systems. The results suggest that PEI may be a promising polymer for use in drug delivery systems due to its ability to disrupt the lipid bilayer and facilitate the release of therapeutic agents. However, the use of PEI also raises concerns about its potential toxicity, highlighting the need for further studies to fully understand its effects on biological membranes. Additionally, experimental studies should be conducted to validate the findings of this study and to further understand the effects of polymer-lipid interactions on biological membranes.
![Order parameter profile of lipid in membrane interacted with polymers. a, Free membrane; b, cellulose; c, chitosan; d, polyethyleneimine (PEI); e, alginate; and f, polylactic-co-glycolic acid (PLGA), [A, sn-1 chain; and B, sn-2 chain] Order parameter profile of lipid in membrane interacted with polymers. a, Free membrane; b, cellulose; c, chitosan; d, polyethyleneimine (PEI); e, alginate; and f, polylactic-co-glycolic acid (PLGA), [A, sn-1 chain; and B, sn-2 chain]](https://services.brieflands.com/cdn/serve/3170b/a83fc1a4185d24985be4828b9f423a72157de39a/jrps-159389-i006-F6-preview.webp)
![Lateral diffusion of lipids in bilayers for a, dipalmitoylphosphatidylcholine (DPPC); b, dipalmitoylphosphatidylethanolamine (DPPE); c, 1-palmitoyl-2-oleoylphosphatidylcholine (POPC); d, 1-palmitoyl-2-oleoylphosphatidylethanolamine (POPE); e, 1-stearoyl-2-oleoylphosphatidylcholine (SOPC); f, 1-stearoyl-2-oleoyl-phosphatidylethanolamine (SOPE); g, 1-stearoyl-2-oleoyl phosphatidylglycerol (SOPG); and h, stearoyloleoylphosphatidylserine (SOPS) in interaction between polymers. [A, Free membrane; B, cellulose; C, chitosan; D, polyethyleneimine (PEI); E, alginate; and F, polylactic-co-glycolic acid (PLGA)]. Lateral diffusion of lipids in bilayers for a, dipalmitoylphosphatidylcholine (DPPC); b, dipalmitoylphosphatidylethanolamine (DPPE); c, 1-palmitoyl-2-oleoylphosphatidylcholine (POPC); d, 1-palmitoyl-2-oleoylphosphatidylethanolamine (POPE); e, 1-stearoyl-2-oleoylphosphatidylcholine (SOPC); f, 1-stearoyl-2-oleoyl-phosphatidylethanolamine (SOPE); g, 1-stearoyl-2-oleoyl phosphatidylglycerol (SOPG); and h, stearoyloleoylphosphatidylserine (SOPS) in interaction between polymers. [A, Free membrane; B, cellulose; C, chitosan; D, polyethyleneimine (PEI); E, alginate; and F, polylactic-co-glycolic acid (PLGA)].](https://services.brieflands.com/cdn/serve/3170b/0dc814c73d4672c782c3af5ebf61e3bed3911b46/jrps-159389-i007-F7-preview.webp)