1. Background
Outbreaks of respiratory diseases are a major cause of global health concerns, resulting in significant rates of morbidity and mortality. Among the most frequently encountered conditions affecting the respiratory system are asthma, bronchitis, the common cold, viral infections, and coughs of varying severity. The cough reflex is a prevalent respiratory symptom and functions as a physiological defense mechanism. It often subsides once the airways are free of excessive secretions; however, a persistent cough can significantly and adversely affect an individual's daily activities, indicating the need for medical intervention.
The epidemiology of cough can be classified into two distinct categories: Acute and chronic. Acute cough is typically short-lived, often caused by upper respiratory tract infections, and generally subsides over a period of 2 to 3 weeks. In contrast, chronic cough is defined as a cough that persists for more than 8 weeks in adults and children aged 15 and older, or for more than 4 weeks in children under 15 years of age (1, 2).
Chronic cough significantly affects socio-economic factors by diminishing quality of life (QOL) through sleep disturbances, fatigue, urinary stress incontinence, and ultimately leading to reduced productivity (3). In patients with more severe conditions, chronic cough may lead to complications such as rib fractures, pneumothorax, pneumomediastinum, and subcutaneous emphysema. Nevertheless, timely and precise diagnosis of the underlying cause of the cough is crucial for successful treatment (4).
2. Objectives
Antitussives serve as therapeutic agents aimed at providing symptomatic relief for dry and non-productive coughs, each offering distinct advantages and disadvantages. As there is an increasing shift towards natural remedies, antitussives sourced from botanical origins may offer comparable efficacy while potentially minimizing the adverse effects commonly linked to conventional cough suppressants. This research evaluated the effectiveness of an herbal syrup as a treatment for chronic cough and its impact on health-related QOL, and assessed how the syrup affected the overall well-being and daily functioning of these patients. Acute and chronic systemic toxicity of the finished products were also evaluated before the clinical study.
3. Methods
3.1. Preclinical Studies
3.1.1. Experimental Animals and Housing Conditions for Evaluation of Acute and Chronic Systemic Toxicity of Finished Product
Male NMRI mice weighing 25 - 30 g were housed and provided with water and standard pellets from an authorized supplier, with a 24-hour fasting period prior to oral administration. Healthy mice were acclimatized to laboratory conditions before the treatment and were then individually housed in stainless steel suspended cages. Systemic toxicity was evaluated in vivo according to the ISO 10993-11 standard by Nikopharmed Aria Co.
3.2. Clinical Study
3.2.1. Study Design
This phase III, randomized, double-blind, placebo-controlled trial was conducted at the 16-Azar Clinic (Tehran, Iran) between July 15 and August 30, 2024. The primary objective of the trial was to assess the efficacy of an herbal syrup in patients suffering from chronic cough.
3.2.2. Ethics and Trial Registration
The study protocol received ethical approval from Tehran University of Medical Sciences (ethics ID: IR.TUMS.TIPS.REC.1403.058). Additionally, the trial was registered in the Iranian Clinical Trials Registry under registration number IRCT20240703062321N1. Before participating in the trial, each patient or their authorized representative was required to sign a form indicating informed consent. Furthermore, the principal investigator and/or co-principal investigator provided a verbal explanation of the essential information relevant to the trial. Demographic information and medical histories of each patient were recorded.
3.2.3. Inclusion Criteria
This study included patients who met the following specific criteria: Participants aged 18 to 80 years, regardless of gender. Eligible patients included those with unexplained chronic cough without an associated diagnosis, or a persistent cough that had not subsided following previous viral infections. The study also included individuals who experienced coughs after receiving treatment for conditions related to asthma, rhinitis, or gastroesophageal reflux disease, including those taking angiotensin-converting enzyme inhibitors. Additionally, only patients — or their legal representatives — who signed the informed consent form were enrolled in the study.
3.2.4. Exclusion Criteria
Patients who met the following criteria were excluded from the trial: Exhibiting any form of hypersensitivity, such as allergies, hives, or skin rashes, to the herbal syrup or any of its ingredients such as eucalyptus, thyme, Malva sylvestris, green tea, Althaea officinalis, ginger, Hyssopus officinalis, purple coneflower, garlic, Marrubium vulgare, and peppermint; any gastrointestinal symptoms caused by the herbal syrup including nausea, diarrhea, and vomiting; patients with a history of gastrointestinal bleeding; pregnancy or breastfeeding; cough with infectious sputum; patients who have been diagnosed with a significant mental or neurological disorder; patients scheduled for possible surgery within the next two weeks; diabetic patients exhibiting uncontrolled blood sugar levels over the past three months; and those who refused to sign informed consent.
3.3. Randomization and Blinding
In this study, participants were randomly divided into two groups: One receiving herbal syrup and the other receiving a placebo. Based on previous studies (5), the sample size was determined using Cochran’s formula. In this formula, the parameters p and q are both set to 0.5. The variable Z represents the normal distribution value corresponding to a confidence level of α-1. For a two-tailed test at a 95% confidence level, the value of Z is 1.96. The variable d represents the desired level of precision, or the margin of error. N is the size of the population in the previous studies. The sample size was estimated to be 120 patients (60 patients in the treatment group and 60 patients in the placebo group), considering a 20% drop rate.
A total of 120 patients were assigned a number ranging from 1 to 120, with the randomization process done using random.org/integers. The numbers in the first column were assigned to group A, while those in the second column were allocated to group B. To determine the assignment of each group to either the intervention or control condition, a lottery method was employed. Throughout the duration of the trial, both researchers and participants remained unaware of the specific treatment assignments, thereby preserving the double blinding of the study.
3.4. Intervention
Following the acquisition of written consent from the participants, patients were assigned to one of the trial's arms through the permutation block randomization method. The treatment group received herbal syrup three times a day along with standard treatment for 10 days, and the control group received a placebo along with the same standard treatment for the same period. Standard treatment provided to all participants included the administration of montelukast (10 mg once daily) and N-acetylcysteine (600 mg effervescent tablets twice daily for 10 days). The quantities of all the plants in this formulation are equal, with the exception of eucalyptus and ginger, which are present at half the amount compared to the other ingredients.
3.5. Clinical Measurements and Assessment of the Outcome
Upon the enrollment of participants in the study, demographic information along with various health conditions, including smoking habits, allergies, viral infections, and the duration of cough, were carefully documented in the case report form (CRF). The treatment regimen was established to last for a period of ten days, with a follow-up scheduled for ten days post-treatment. The patients were contacted for follow-up assessments on the third and twentieth days, during which they were inquired about their adherence to the prescribed medications. Each participant completed the Leicester Cough Questionnaire (LCQ) at two distinct time points: At baseline and on the twentieth day of the study. This validated instrument consists of 19 self-reported items, each rated on a 7-point Likert scale, designed to evaluate the impact of cough on QOL over the preceding two weeks. The LCQ measures the physical, social, and psychological consequences of chronic cough, with higher scores reflecting a more favorable health status. The primary objective of the study was to observe an enhancement in the LCQ scores throughout the twenty-day treatment duration, while the secondary aim focused on evaluating the QOL associated with chronic cough as indicated by the LCQ results. An increase in the scores was interpreted as a sign of improved health outcomes for the participants, thereby indicating the importance of the treatment in alleviating the burden of chronic cough in their daily lives.
3.6. Statistical Analysis
Quantitative data were expressed as mean ± standard deviation, with a significance threshold set at P-value < 0.05. To analyze the demographic characteristics, the chi-square test was employed for categorical variables, while the Mann-Whitney U test and the t-test were utilized for quantitative variables.
4. Results
4.1. Evaluation of Acute Systemic Toxicity of Finished Product
During the study period, all mice in both the treatment and control groups survived, and no mortality was recorded. Furthermore, none of the animals displayed any unusual clinical symptoms that would suggest the presence of toxicity. This observation is noteworthy because it indicates the absence of adverse effects during the experimental period. Body weight fluctuations observed in the animals during the experimental phase were within normal limits. Overall, these results indicated that the consistent health status of the mice, alongside the absence of clinical signs of toxicity, suggested no acute toxicity of the final product at the prescribed dose of 50 mL/kg.
4.2. Evaluation of Chronic Systemic Toxicity of Finished Product
Until the necropsy, none of the tested animals exhibited abnormal clinical signs indicative of toxicity during the test period. All the animals remained alive throughout the duration of the test. Body weight and organ weight fluctuations stayed within normal physiological ranges during the study. None of the tested animals exhibited abnormal clinical pathology, gross pathology, or histopathological signs indicative of toxicity during the test period. The results support the notion that the examined product does not induce chronic systemic toxicity. Histopathological examinations of the vital organs (kidney and liver) of all mice exposed to chronic treatment with the herbal syrup showed no apparent lesions or structural alterations at the dose of 50 mg/kg after 6 months (Appendix 1 in Supplementary File).
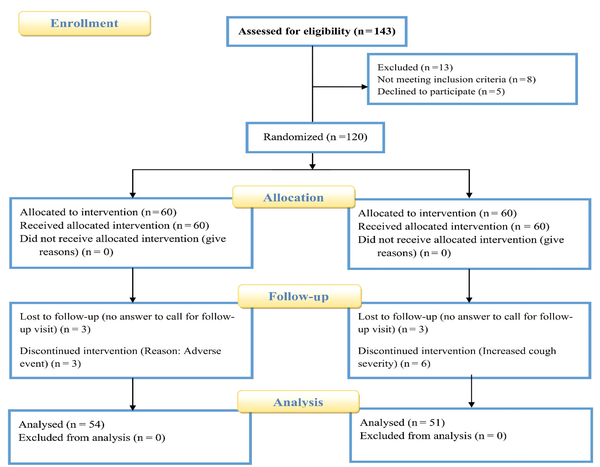
4.3. Demographic Details and Patient Enrollment Summary
From July 15, 2024, to August 30, 2024, a total of 143 individuals suffering from chronic cough were assessed for their eligibility to participate in the study. Out of these, 120 patients met the criteria for inclusion. Ultimately, 105 patients proceeded to enroll in the study, with 54 individuals allocated to the intervention group and 51 to the placebo group. During the study period, five participants chose to withdraw voluntarily due to issues such as incorrect contact information and a lack of response during follow-up visits. Additionally, ten patients discontinued their participation due to adverse effects experienced in the treatment group or an exacerbation of cough severity in the placebo group. A CONSORT flow diagram of the study is depicted in Figure 1. The age of the patients in the treatment group was 43.47 ± 17.96 years, with 53.7% male participants. In contrast, the placebo group had an average age of 46.31 ± 14.58 years, and 51% of the participants were male. Statistical analysis revealed no significant difference in age between the two groups (P-value = 0.37). There were no significant differences among the basic characteristics, including gender, body weight, height, duration of cough, viral diseases, smoking status, and allergies (Table 1).
| Demographic Features | Treatment Group (n = 54) | Placebo Group (n = 51) | P-Value |
|---|---|---|---|
| Gender | 0.780 | ||
| Male | 29 (53.7) c | 26 (51) c | |
| Female | 25 (46.3) c | 25 (49) c | |
| Age (y) | 43.43 ± 17.96 d | 46.31 ± 14.58 d | 0.370 |
| Body weight (kg) | 70.81 ± 14.79 d | 72.78 ± 12.29 d | 0.461 |
| Height (cm) | 170.28 ± 10.31 d | 169.20 ± 7.73 d | 0.690 |
| Duration of cough | 22.18 ± 64.40 d | 30.35 ± 57.15 d | 0.736 |
| Smoke | 13 (24.1) c | 13 (25.5) c | 0.867 |
| Viral disease | 47 (87) c | 43 (84.3) c | 0.692 |
| Allergy | 13 (24.1) c | 19 (37.3) c | 0.093 |
4.4. Efficacy
The scores of each of the 19 questions related to the LCQ form were compared between the treatment and placebo groups at baseline and at the end of the follow-up period, as listed in Appendix 2 in Supplementary File. There was no significant difference in the score of each question between the groups at baseline, except for question 7, in which patients in the placebo group experienced coughs that interfered with their work or other daily tasks more than those in the treatment group. Following treatment with herbal syrup, patients exhibited significant improvement in various health parameters, including sputum production decline (P-value = 0.000), fatigue levels (P-value = 0.002), cough control (P-value = 0.042), overall life satisfaction (P-value = 0.01), sleep quality (P-value = 0.011), frequency of coughing episodes (P-value = 0.017), and occurrences of a hoarse voice (P-value = 0.002) compared to the control group. While in other conditions such as chest or stomach pain, sensitivity to paints or fumes, anxiety, frustration, anorexia, energy level, etc., no significant improvement was observed between the treatment group and the placebo group (P-value > 0.05) (Appendix 2 in Supplementary File).
At baseline, the total LCQ scores were recorded as 10.49 ± 2.66 for the placebo group and 11.12 ± 2.56 for the treatment group. Although the treatment group initially had higher scores than the control group, this difference was not statistically significant (P-value = 0.225). At the end of the follow-up period, both groups demonstrated an increase in their total LCQ scores. However, the treatment group experienced more significant improvement after the intervention (13.46 ± 2.40) than the placebo group (11.91 ± 2.43). This increase in scores was statistically significant, indicating that the treatment had a beneficial effect on participants' outcomes (P-value < 0.001) (Table 2).
| LCQ Domains, Time Points and Groups | No. | Mean ± SD | Effect Size | P-Value |
|---|---|---|---|---|
| Total LCQ | ||||
| Baseline | 0.237 | 0.225 | ||
| Treatment | 54 | 11.12 ± 0.34 | ||
| Placebo | 51 | 10.49 ± 0.37 | ||
| Post-Tx | 0.635 | < 0.001 b | ||
| Treatment | 54 | 13.46 ± 0.32 | ||
| Placebo | 51 | 11.91 ± 0.34 | ||
| Physical | ||||
| Baseline | 0.192 | 0.328 | ||
| Treatment | 54 | 4.16 ± 0.14 | ||
| Placebo | 51 | 3.95 ± 0.14 | ||
| Post-Tx | 0.739 | < 0.001 b | ||
| Treatment | 54 | 5.29 ± 0.14 | ||
| Placebo | 51 | 4.48 ± 0.15 | ||
| Psychological | ||||
| Baseline | 0.127 | 0.192 | ||
| Treatment | 54 | 4.61 ± 0.14 | ||
| Placebo | 51 | 4.38 ± 0.16 | ||
| Post-Tx | 0.441 | 0.018 c | ||
| Treatment | 54 | 5.47 ± 0.13 | ||
| Placebo | 51 | 5.03 ± 0.14 | ||
| Social | ||||
| Baseline | 0.262 | 0.186 | ||
| Treatment | 54 | 2.34 ± 0.09 | ||
| Placebo | 51 | 2.15 ± 0.1 | ||
| Post-Tx | 0.484 | 0.003 d | ||
| Treatment | 54 | 2.69 ± 0.07 | ||
| Placebo | 51 | 2.4 ± 0.08 |
Leicester Cough Questionnaire Scores: Baseline and Post-treatment Comparison a
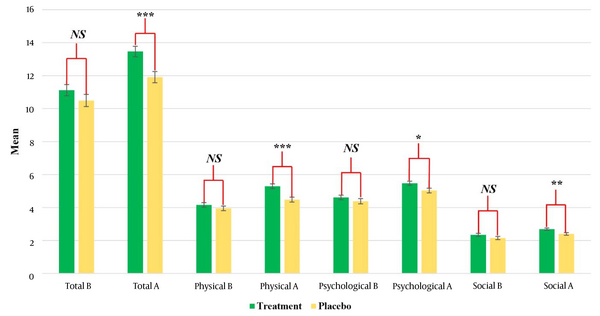
The LCQ evaluates three domains: Physical, social, and psychological (Figure 2). Baseline scores for these domains showed no significant differences between groups. The administration of herbal syrup resulted in a statistically significant increase in physical (P-value < 0.001), social (P-value = 0.018), and psychological (P-value = 0.003) scores when compared to the placebo group, with measurements taken ten days post-treatment cessation. These findings suggest that the beneficial antitussive properties of herbal syrup remained even after its discontinuation. The placebo group exhibited an increase in scores related to physical, social, and psychological effects; however, these changes were not statistically significant when compared to the treatment group. Overall, the data analysis revealed that the administration of herbal syrup had considerable effects on LCQ scores in the three determined subgroups (Table 2 and Figure 2).
Mean Leicester Cough Questionnaire (LCQ) scores for total, physical, psychological, and social domains at baseline (B) and post-treatment (A) in the treatment and placebo groups. Error bars indicate standard error of the mean. Statistical significance is denoted as: * P < 0.05, ** P < 0.01, *** P < 0.001; Abbreviation: NS, not significant.
4.5. Subgroup Analysis
Approximately fifty percent of the patients reported experiencing viral infections in recent months. Therefore, a subgroup analysis was conducted to differentiate between patients with viral infections and those without (Table 3). The results revealed that the use of herbal syrup led to a statistically significant enhancement in total (P-value < 0.001), physical (P-value < 0.001), social (P-value = 0.008), and psychological (P-value = 0.019) scores among patients suffering from viral illnesses, in comparison to the placebo group that also had a background of viral diseases. The analysis underscores the importance of evaluating the effects of herbal syrup on patients with viral infections. The significant improvements in various health scores suggest that herbal interventions may offer beneficial outcomes for individuals affected by such conditions, highlighting the potential for alternative treatment options in managing viral-related health issues.
| LCQ Domain, Viral Diseases and Time Points | P-Value |
|---|---|
| Total LCQ | |
| Baseline | |
| Yes | 0.129 |
| Treatment | |
| Placebo | |
| No | 0.62 |
| Treatment | |
| Placebo | |
| Post-Tx | |
| Yes | < 0.001 b |
| Treatment | |
| Placebo | |
| No | 0.463 |
| Treatment | |
| Placebo | |
| Physical | |
| Baseline | |
| Yes | 0.122 |
| Treatment | |
| Placebo | |
| No | 0.536 |
| Treatment | |
| Placebo | |
| Post-Tx | |
| Yes | < 0.001 b |
| Treatment | |
| Placebo | |
| No | 0.121 |
| Treatment | |
| Placebo | |
| Psychological | |
| Baseline | |
| Yes | 0.15 |
| Treatment | |
| Placebo | |
| No | 0.518 |
| Treatment | |
| Placebo | |
| Post-Tx | |
| Yes | 0.019 c |
| Treatment | |
| Placebo | |
| No | 0.901 |
| Treatment | |
| Placebo | |
| Social | |
| Baseline | |
| Yes | 0.131 |
| Treatment | |
| Placebo | |
| No | 0.726 |
| Treatment | |
| Placebo | |
| Post-Tx | |
| Yes | 0.008 d |
| Treatment | |
| Placebo | |
| No | 0.961 |
| Treatment | |
| Placebo |
Subgroup Analysis: Leicester Cough Questionnaire Scores by Viral Disease History a
4.6. Safety and Adverse Events
The potential interactions of each herb were evaluated according to different conditions and standard treatments listed in the NatMed database (6). The findings revealed that there were no significant interactions identified between these herbs and the corresponding medications. During the trial period and the subsequent follow-up period, no significant adverse effects were recorded. However, among the patients who were excluded from the study, two patients experienced symptoms of heartburn and acid reflux, while one patient reported the appearance of red spots on the skin, which were attributed to an allergy to the prescribed herbal syrup. The aforementioned conditions were transient in nature and stopped after discontinuation of the herbal syrup.
5. Discussion
With high rates of morbidity and mortality, respiratory illness outbreaks are a leading cause of global health issues. Asthma, bronchitis, the common cold, viral infections, and coughs of various intensities are among the most prevalent respiratory system ailments. This study was conducted as a randomized, double-blind, placebo-controlled clinical trial from July 15, 2024, to August 30, 2024. A total of 120 individuals suffering from chronic cough participated in this study. According to the findings, patients with viral illnesses who used herbal syrup had statistically significant improvements in their overall (P-value < 0.001), physical (P-value < 0.001), social (P-value = 0.008), and psychological (P-value = 0.019) scores when compared to the placebo group, which also had a history of viral diseases. The primary outcome was assessed using the LCQ, which measures the QOL related to chronic cough. The findings indicated that participants who received herbal syrup experienced notable enhancements in several health metrics. Furthermore, the administration of herbal syrup significantly influenced the physical, social, and psychological scores of the participants. Overall, the treatment led to an improvement in the QOL for the patients and a reduction in the frequency of cough episodes within the treated group. These findings underscore the potential benefits of herbal syrup in managing chronic cough and enhancing patients' QOL.
Numerous clinical investigations have explored the therapeutic potential of combining various medicinal plants in the management of cough. This study evaluated the therapeutic potential of a herbal syrup containing eleven medicinal plants for alleviating chronic cough symptoms. The primary mucolytic monoterpene in the leaves of eucalyptus is eucalyptol, which has been extensively investigated in both preclinical and clinical research. Eucalyptol exhibits a range of pharmacological properties, including anti-inflammatory, antiviral, antioxidant, bronchodilatory, and antimicrobial activities, which can explain its beneficial effects in the treatment of respiratory disorders. Numerous clinical studies have investigated the therapeutic effects of eucalyptus on patients suffering from respiratory conditions, which have yielded encouraging outcomes (7, 8). Eucalyptol promoted activation of interferon regulatory factor 3 (IRF3), modulation of NF-κB activity, and the downregulation of mucin genes (MUC2 and MUC19), LTB4, IL-1β, IL-6, IL-8, and TNF-α, while regulating Th1/2-type inflammatory pathways (9, 10).
Althaea officinalis, commonly known as marshmallow, has a long history of use in alleviating cough and various respiratory ailments. The soothing and protective properties of its mucilage components are particularly beneficial for inflamed mucous membranes in the upper respiratory tract, which can relieve coughs. In such instances, A. officinalis is often utilized as an antitussive agent. Furthermore, when combined with other herbal remedies such as Zingiber officinalis, the therapeutic potential of A. officinalis is significantly enhanced. This synergistic effect highlights the importance of exploring herbal combinations in respiratory care to optimize treatment efficacy (11, 12). The bioactive compounds found in ginger extract, including 6-gingerol, 8-gingerol, and 6-shogaol, exhibit significant antioxidant and anti-inflammatory properties. These compounds play a crucial role in mitigating inflammatory responses by targeting various inflammatory mediators, including cytokines and chemokines, thereby enhancing the body's ability to reduce inflammation (13, 14).
Hyssopus officinalis L. has been recognized for its potential therapeutic effects in alleviating cough and asthma symptoms by modulating the secretion levels of interleukins IL-4, IL-6, IL-17, and interferon-γ (IFN-γ), and correcting the imbalance between Th1 and Th2 cell responses (15, 16). Furthermore, H. officinalis may contribute to the prevention of airway remodeling by restoring the balance of the matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinases-1 (TIMP-1) ratio. This regulatory effect highlights the herb's potential role in maintaining respiratory health and mitigating chronic inflammatory conditions associated with the airways (17).
Echinacea contains several bioactive compounds, including flavonoids, alkylamides, polysaccharides, chicoric acid, polyacetylenes, and essential oils. Research has indicated that Echinacea exhibits both antiviral and antibacterial effects, supporting its application in the prevention and management of upper respiratory tract infections. The immunomodulatory properties of Echinacea are thought to underlie its beneficial effects on health, particularly in boosting the immune response. Various studies have explored its efficacy in mitigating symptoms associated with respiratory illnesses, suggesting that the plant may play a significant role in enhancing overall immune resilience (18, 19).
Pimpinella anisum L., commonly known as anise, is utilized not only in the management of gastrointestinal disorders but also in the treatment of ailments related to the upper respiratory system, as well as fever and inflammation affecting the oral cavity and throat. The seeds possess aromatic compounds and exhibit expectorant-like effects, which can be beneficial for the lungs and airways. In traditional medicine, anise is administered internally for conditions like bronchial catarrh, asthma, spasmodic cough, pertussis, and flatulent colic, while it is applied externally for treating pediculosis and scabies. Research has demonstrated a statistically significant bronchodilatory effect of anise essential oil, as well as its aqueous and ethanol extracts, in various animal models (20, 21). Additionally, aniseed essential oil has been shown to diminish the levels of pro-inflammatory cytokines, specifically IL-1β and IL-8, indicating the anti-inflammatory effects of this medicinal plant (22).
Numerous clinical investigations have explored the therapeutic potential of combining various medicinal plants in the management of cough. Findings from these studies indicate that the administration of herbal syrups is effective in alleviating symptoms in patients suffering from chronic cough (5, 23-25). Carnevali et al. also showed that KalobaTUSS®, a pediatric cough syrup based on medicinal plants such as acacia honey and M. sylvestris extract, Inula helenium extract, Plantago major extract, and Helichrysum stoechas extract, provided beneficial effects by reducing the severity and shortening the duration of cough in children (26).
The results of the present study demonstrated the significant effectiveness of the administered herbal syrup in the treatment of patients with chronic cough and showed its role as an alternative therapeutic approach in the treatment of cough.