1. Background
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune disease that primarily impacts the central nervous system, especially the optic nerves and spinal cord (1, 2). The neuromyelitis optica (NMO)-IgG antibody specifically targets aquaporin-4 (AQP4), a water-channel protein that forms homotetramers in cell membranes and is essential for maintaining fluid balance in the central nervous system (3). Although AQP4 is found throughout the brain, it is especially abundant in the optic nerves and spinal cord (4). The presence of AQP4 antibodies (AQP4-Ab) plays a significant role in the development of NMOSD, likely through a process that involves complement-mediated damage to astrocytes. This leads to the infiltration of leukocytes, the release of cytokines, and a breakdown of the blood-brain barrier, ultimately causing injury to oligodendrocytes, loss of myelin, and neuronal injury (5). Notably, the pathophysiology of NMOSD differs between AQP4-positive and AQP4-negative patients, as the latter group may involve alternative immune mechanisms, such as myelin oligodendrocyte glycoprotein (MOG) antibodies, or other unidentified pathways (6).
Historically, it was believed that NMO does not affect the brain, and a negative brain MRI at the onset of the disease was once a crucial diagnostic criterion. However, a compelling body of evidence shows that brain MRIs often demonstrate lesions in patients with NMOSD (7). Asymptomatic brain lesions are commonly found, but symptomatic brain involvement is also frequent, and in some cases, brain symptoms may be the first sign of NMOSD (8). Acute brain abnormalities in NMOSD are usually identified as T2-weighted or fluid attenuated inversion recovery (FLAIR) hyperintense signals (9). Many brain lesions associated with NMO or NMOSD differ from those seen in multiple sclerosis (MS), with specific lesion patterns often corresponding to areas of high AQP4 expression, such as regions near the ventricular system (10). However, NMO-specific lesions have also been identified in areas where AQP4 expression is not significantly elevated (11).
While brain and spinal MRIs are crucial for diagnosing NMOSD, the role of brain MRI is increasingly acknowledged for its importance in predicting outcomes. Understanding lesion distribution and burden could have significant implications for prognosis, as lesion location may influence disability progression and long-term neurological outcomes. Moreover, identifying patterns of brain involvement could refine treatment strategies by guiding personalized therapeutic approaches for different NMOSD subtypes.
The Expanded Disability Status Scale (EDSS) is a well-known tool for evaluating disability (12). Clinicians use this scale to evaluate different functional systems of the central nervous system, and it is commonly employed to track disease progression in patients with MS as well as to evaluate treatment efficiency in clinical trials (13). The EDSS features an ordinal rating system that ranges from 0, which indicates normal neurological function, to 10, which signifies death due to MS, with increments of 0.5 starting at EDSS 1. Lower EDSS scores indicate neurological impairments identified through clinical examination, while scores above 6 primarily reflect functional disabilities (14). Although the EDSS has conventionally focused on assessing MS-related motor disabilities, clinicians are increasingly using this scoring system for NMOSD as well (15). However, the factors influencing EDSS progression in NMOSD remain less clearly defined compared to MS (16). Specifically, the relationship between lesion burden and functional disability in NMOSD, particularly regarding brain involvement, requires further investigation. Given that lesion patterns may differ based on AQP4 status, a better understanding of their impact on EDSS could provide insights into disease severity and guide therapeutic decision-making.
The link between brain lesions and disability status has been extensively studied in MS (17). However, this connection is less clearly established in NMO. While the mechanisms behind brain lesions in MS, such as demyelination, are different from those in NMO, which involves astrocytopathy and oligodendrocytopathy (18), previous studies have provided limited data on how lesion burden correlates with clinical disability in NMOSD. Some studies have suggested that brain lesions contribute to disease severity, but findings remain inconsistent (19), and most research focused on spinal cord involvement as the primary determinant of disability. Moreover, studies often do not differentiate between AQP4-positive and AQP4-negative patients, despite the potential differences in lesion pathology and clinical outcomes.
2. Objectives
This study specifically assessed T2/FLAIR hyperintense brain lesions in NMOSD and their correlation with EDSS, with a particular focus on comparing AQP4-positive and AQP4-negative patients. It emphasizes the distribution of these lesions and their diverse impacts on disability, aiming to clarify the role of brain involvement in NMOSD progression. By distinguishing lesion patterns between antibody subgroups, our findings could enhance the clinical understanding of NMOSD and inform prognosis, patient management, and potential therapeutic strategies.
3. Patients and Methods
3.1. Study Population
A previously established list of patients with a confirmed diagnosis of NMOSD was obtained from the MS Research Center’s NMO dataset at Sina Hospital (Tehran University of Medical Sciences, Tehran, Iran), a tertiary referral institute. The diagnosis of NMOSD was made based on international consensus diagnostic criteria for neuromyelitis optica spectrum disorders (20). While overlapping with a dataset used in our prior study (21) focused on T1 hypointense lesions, the current analysis uniquely examines T2/FLAIR hyperintense lesions, providing complementary insights into NMOSD lesion characteristics and their relationship with EDSS.
The demographic data, disease characteristics, EDSS, and laboratory results (anti-AQP4 antibody) were obtained from archived inpatient and outpatient records. The study population was then divided into two groups based on AQP4 status. Afterward, the documented data were cross-checked to verify consistency. This study adhered to the ethical guidelines outlined in the Helsinki Declaration (22) and was approved by the Research and Ethics Committee of the Sina Hospital MS Research Center. Additionally, patients had provided informed consent at the time of admission or consultation for the future use of their anonymized data.
3.2. Laboratory Study
A fixed cell-based assay (CBA) test was used to detect AQP4 antibodies in serum. Patients were categorized into two groups: AQP4 positive and AQP4 negative.
3.3. Image Analysis
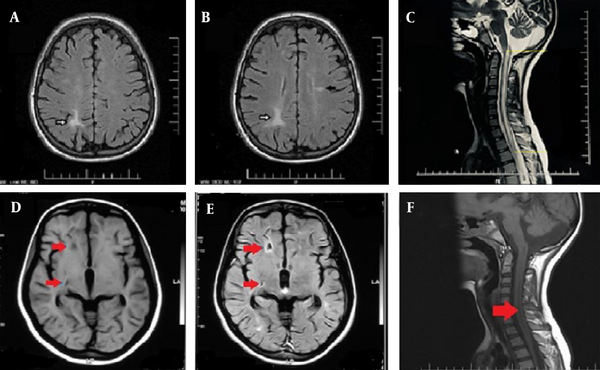
The latest brain and spinal MRIs for each patient were evaluated, with all scans conducted on 1.5 Tesla machines. An expert radiologist reviewed the images using the INFINITT PACS System (INFINITT Healthcare Co. Ltd., Seoul, South Korea). This study characterized T2/FLAIR hyperintense lesions in the brain and spinal cord that displayed high T2 and normal T1 signal intensities, measuring 3 mm or more (Figure 1).
Brain axial fluid attenuated inversion recovery (FLAIR) (A, B); and cervical cord sagittal T2-weighted (C); MRI sequences of a 22-year-old aquaporin-4 (AQP4) positive male patient with neuromyelitis optica (NMO) diagnosis and no other noticeable medical history. Hemispheric white matter (A and B, white arrows) and lateral ventricle’s periependymal (B, black arrow) lesions depicted as FLAIR hyperintensities. Concurrent spinal cord involvement as continuous, long segment T2 hyperintensity is also illustrated (C). Brain axial T1 (D); and FLAIR (E) sequences; and cervical spine sagittal T1 (F) planes of a 23-year-old female patient with NMO (AQP4-positive) diagnosis. MR images depict hemispheric white matter lesion as T1-hypointense lesions (arrows). Spinal MRI shows long segment involvement of the cervical cord.
Neuromyelitis optica -related brain lesions were categorized in the following areas: Hemispheric white matter, corticospinal tract (CST), periependymal regions surrounding the third and fourth ventricles/aqueduct, periependymal regions adjacent to the lateral ventricles, corpus callosum, and superior tegmentum. Lesions located in sites other than the aforementioned areas were labeled as "others." In addition to the lesions' location, the number of lesions in each region and the total burden of lesions in the brain were recorded. The length of spinal cord lesions' extension was also measured.
3.4. Statistical Analysis
Data analysis was conducted using SPSS v26 (IBM SPSS Statistics for Windows, version XX-IBM Corp., Armonk, N.Y., USA). Quantitative variables are presented as mean ± standard deviation, whereas qualitative variables are reported as number and percentage. An independent samples t-test and analysis of variance (ANOVA) were applied to compare EDSS scores across different groups. The relationship between AQP4 status and the presence or absence of brain lesions was evaluated using the chi-square test. Additionally, a multiple linear regression model was developed to control for spinal lesion length as a confounding factor in evaluating the association between brain lesions and EDSS scores. The significance level was considered less than 0.05.
4. Results
4.1. Demographic Characteristics
After referring to the Sina Hospital NMO dataset, 82 subjects were eligible to participate in the current study. After excluding subjects who did not meet the inclusion criteria (e.g., lack of adequate lab or imaging data or EDSS), 72 patients [male: N = 15 (20.5%)] were included in the study. The mean age of the participants was 33.38 ± 11.32 years (range: 16 - 65) (Table 1).
| Variables | Whole subjects | NMOSD with positive AQP4; 36 (49.3) | NMOSD with negative AQP4; 37 (50.7) | P-value |
|---|---|---|---|---|
| Age (y) | 33.35 ± 11.27 | 34.7 ± 12.2 | 32.0 ± 10.3 | 0.721 |
| Gender | ||||
| Female | 58 (79.4) | 30 (83.3) | 28 (75.7) | 0.122 |
| Male | 15(20.6) | 6 (16.7) | 9 (24.3) | 0.067 |
| Age at onset (y) | 28.52 ± 9.3 | 30.5 ± 12.1 | 25.6 ± 7.8 | 0.115 |
| Disease duration (y) | 5.2 ± 4.5 | 4.3 ± 3.1 | 6.3 ± 5.6 | 0.075 |
| EDSS | 2.9 ± 1.6 | 3.2 ± 1.7 | 2.7 ± 1.6 | 0.849 |
Demographic Characteristics of the Whole Subjects and in Subgroups of Patients with and Without Aquaporin-4 Antibody a
4.2. Lesion Distribution
Of all 72 participants, 46 (63%) patients had T2/FLAIR hyperintense brain lesions. As depicted in Table 2, a total of 224 T2/FLAIR hyperintense lesions were found, with the majority located in the hemispheric white matter (99 lesions, 44.00%), followed by the periependymal region of the lateral ventricles (65 lesions, 29.01%).
| Location of the lesions | AQP4 positive | AQP4 negative | All participants | P-value |
|---|---|---|---|---|
| Hemispheric white matter | 55 | 42 | 97 | 0.44 |
| Corticospinal tract | 8 | 9 | 17 | 0.88 |
| Periependymal (lateral ventricles) | 26 | 39 | 65 | 0.26 |
| Periependymal (3rd and 4th ventricles) | 14 | 16 | 30 | 0.81 |
| Superior tegmentum | 3 | 1 | 4 | 0.30 |
| Others | 6 | 3 | 9 | 0.09 |
| Total number of lesions | 112 | 112 | 224 | 0.08 |
T2/FLAIR Hyperintense Lesions Frequency in Different Locations Respecting Aquaporin-4 Antibody Status
4.3. Comparison between AQP4-Positive and AQP4-Negative Patients
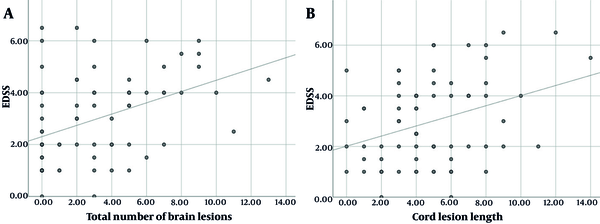
Laboratory data show that 36 (49.8%) patients were AQP4 positive. No association was found between AQP4 status and the presence of brain lesions (Table 3). Mean EDSS was not correlated with AQP4 status (P = 0.849). Among all participants, regardless of AQP4 status, there was a correlation between the EDSS and: (1) The number of hemispheric white matter lesions (r = 0.36, P = 0.002); (2) the number of lateral ventricles periependymal lesions (r = 0.31, P = 0.007); (3) the total number of brain lesions (r = 0.41, P < 0.001); and (4) the length of spinal cord lesion (r = 0.34, P = 0.03) (Figure 2).
| AQP4 and brain lesions | Frequency (%) | P-value |
|---|---|---|
| Negative | 0.41 | |
| No | 12 (32.4) | |
| Yes | 25 (67.6) | |
| Positive | ||
| No | 15 (41.7) | |
| Yes | 21 (58.3) |
Association Between Aquaporin-4 Status and the Presence of Brain Lesions
After splitting subjects into two separate groups with regard to AQP4 status, EDSS was only correlated with CST lesions (r = 0.38, P = 0.018) and the total number of brain lesions (r = 0.37, P = 0.02) in AQP4 negatives. No correlation was observed between EDSS and spinal cord lesion length in this group (P = 0.60).
In the AQP4 positive group, a significant correlation was observed between EDSS and: (1) The number of hemispheric white matter lesions (r = 0.45, P = 0.005); (2) the number of lateral ventricles periependymal lesions (r = 0.37, P = 0.027); (3) the total number of brain lesions (r = 0.45, P = 0.05); and (4) the length of spinal cord lesion (r = 0.61, P < 0.001) (Table 4).
| AQP4 | HWM | CST | LVPE | PE3&4 | ST | Other | Total | Cord lesion length |
|---|---|---|---|---|---|---|---|---|
| Negative (N = 37) | ||||||||
| Pearson correlation | 0.222 | 0.386 | 0.312 | 0.116 | 0.184 | -0.098 | 0.374 | 0.090 |
| Sig. (2-tailed) | 0.187 | 0.018 | 0.060 | 0.496 | 0.276 | 0.565 | 0.023 | 0.598 |
| Positive (N = 36) | ||||||||
| Pearson correlation | 0.455 | 0.042 | 0.369 | 0.054 | 0.044 | 0.315 | 0.455 | 0.607 |
| Sig. (2-tailed) | 0.005 | 0.808 | 0.027 | 0.754 | 0.798 | 0.062 | 0.005 | < 0.001 |
| Total (N = 72) | ||||||||
| Pearson correlation | 0.363 | 0.207 | 0.312 | 0.079 | 0.111 | 0.171 | 0.414 | 0.340 |
| Sig. (2-tailed) | 0.002 | 0.078 | 0.007 | 0.508 | 0.350 | 0.148 | 0.000 | 0.003 |
Correlation Between EDSS and Number of Brain Lesions in Different Locations and Cord Lesion Length
4.4. Regression Analysis Controlling for Confounders
Concerning the potential confounding effect of spinal cord lesion length, a multiple linear regression model was employed to assess the separate effects of brain lesions in hemispheric white matter and lateral ventricles periependymal regions on EDSS in AQP4 positive subjects (Table 5). The number of hemispheric white matter lesions was significantly correlated with EDSS (coefficient = 0.10, P = 0.013). Conversely, the number of lateral ventricles periependymal lesions did not have a separate significant effect (coefficient = 0.18, P = 0.28). Similar analysis demonstrated that the total number of brain lesions is correlated with EDSS in all participants, regardless of the length of cord lesion [the coefficient of regression model variables was 0.05 with a 95% confidence interval (CI); P = 0.001].
| AQP4 | Unstandardized B | Coefficients standard error | Standardized coefficient beta | t | P-value | R square |
|---|---|---|---|---|---|---|
| Negative | ||||||
| Constant | 2.131 | 0.609 | 3.502 | 0.001 | ||
| HWM | 0.086 | 0.204 | 0.081 | 0.420 | 0.677 | |
| LVPE | 0.352 | 0.255 | 0.265 | 1.381 | 0.176 | |
| Cord lesion length | 0.019 | 0.104 | 0.031 | 0.185 | 0.854 | |
| Positive | 0.41 | |||||
| Constant | 1.421 | 0.384 | 3.696 | 0.001 | ||
| HWM | 0.269 | 0.102 | 0.336 | 2.641 | 0.013 | |
| LVPE | 0.199 | 0.180 | 0.145 | 1.109 | 0.276 | |
| Cord lesion length | 0.287 | 0.072 | 0.512 | 3.988 | 0.000 |
Multiple Linear Regression Model Controlled for Cord Lesions’ Length as a Potential Confounder a
5. Discussion
In the current study, we evaluated brain and spinal MRI findings in 72 patients with NMO from the database provided by Sina Hospital MS Center. We provided a descriptive report of T2/FLAIR hyperintense brain and spinal cord lesions and assessed their correlation with the EDSS. The frequency of brain lesions in our study was approximately 63%, consistent with prior studies. Current literature suggests that brain lesions in NMO are more common than previously recognized (8). For instance, studies report that 35 - 50% of NMOSD patients exhibit brain MRI abnormalities, with higher prevalence among those with coexisting autoimmune conditions (23, 24). In another report, during acute attacks, contrast-enhancing brain lesions have been observed in up to 63% of cases (25).
In our study, the most common locations for brain lesions were the hemispheric white matter and the periependymal regions surrounding the lateral ventricles. These results are consistent with previous reports that show NMO lesions typically localize to areas with high AQP4 expression, including periventricular regions, the brainstem, and white matter tracts (26). Earlier research often points to the third and fourth ventricles as more frequently affected areas, likely due to their elevated AQP4 expression (27). This difference might be attributed to variations in patient demographics, disease characteristics, or improvements in imaging techniques that enhance the detection of lesions in lateral periventricular regions. In fact, another recent study reported a higher prevalence of lesions in the periependymal regions around the lateral ventricles (27).
Interestingly, our study did not reveal a significant link between AQP4 antibody status and EDSS. While numerous studies indicate that being positive for anti-AQP4 antibodies correlates with higher EDSS scores in patients with NMO (28, 29), some research disputes this relationship. For instance, a study analyzing clinical parameters in NMOSD patients found no notable differences in EDSS scores between those who were AQP4-positive and those who were AQP4-negative (29, 30). Additionally, another investigation pointed out that while higher AQP4 antibody titers were related to increased relapse rates, they did not show a direct correlation with EDSS or the progression of long-term disability (31). These inconsistencies suggest that the relationship between AQP4 antibodies and disability severity, as measured by EDSS, may be more intricate than previously thought. Various clinical and imaging factors, such as lesion location, the extent of astrocytic damage, and disease phenotype, could play a role in this correlation. Therefore, further research is needed to clarify how AQP4 antibodies contribute to neurological disability and to determine whether other biomarkers or parameters could better predict disease progression and severity.
Among all participants, regardless of AQP4 antibody status, a significant correlation was observed between EDSS and the length of spinal cord lesions, the total number of brain lesions, and specific brain regions, such as hemispheric white matter and lateral ventricular periependymal areas. However, subgroup analysis revealed different correlation patterns based on AQP4 status. In AQP4-negative patients, the EDSS was found to correlate with lesions in the CST and the overall number of brain lesions, but it did not correlate with the length of spinal cord lesions. Conversely, AQP4-positive patients exhibited significant correlations between EDSS and the total number of brain lesions, the length of spinal cord lesions, the count of hemispheric white matter lesions, and lesions around the lateral ventricular periependymal tissue.
Notably, the length of spinal cord lesions was correlated with EDSS only in the AQP4-positive group, while CST lesions were significantly linked to EDSS exclusively in the AQP4-negative group. Interestingly, the total number of brain lesions consistently showed a positive correlation with EDSS in both patient groups. These findings emphasize the different roles that lesion distribution and burden have in the progression of disability among AQP4-positive and AQP4-negative patients. For AQP4-positive individuals, spinal cord involvement seems to be the main factor leading to disability, whereas lesions in the CST appear to be more important for AQP4-negative patients. The total number of brain lesions, irrespective of their specific locations, may act as a general indicator of disease burden and its effect on disability progression in both groups.
The observed connection between the EDSS and spinal cord lesion length in AQP4-positive patients, but not in AQP4-negative patients, underscores key differences in the underlying pathophysiology of NMO related to antibody status. In AQP4-positive patients, the presence of anti-AQP4 antibodies causes damage to astrocytes through complement-mediated processes, leading to severe inflammation and extensive spinal cord lesions (32). These lesions are often associated with considerable demyelination and axonal injury, which can hinder recovery and result in greater disability (33). This mechanism aligns with the strong relationship observed between lesion length and EDSS in AQP4-positive individuals.
On the other hand, the clinical progression in AQP4-negative patients is more varied, often presenting with milder or atypical forms of NMOSD that do not profoundly depend on spinal cord lesion length to assess disability (34, 35). Other elements, such as the burden of brain lesions, involvement of the CST, or different pathogenic mechanisms (like MOG antibody-associated disease), may have a more pronounced impact on their disability progression. Additionally, the repair processes in AQP4-negative patients might differ, potentially allowing for better remyelination or less axonal damage, which could lessen the effect of spinal cord lesion length on EDSS.
These distinctions emphasize the necessity for antibody-specific strategies in understanding and treating NMO, as the pathophysiological differences between AQP4-positive and AQP4-negative patients may require customized therapeutic approaches.
It is important to highlight that the CST is not a region with high expression of AQP4, and the frequent involvement of this area in AQP4 autoimmunity has been a subject of debate (8). In our study, we did not find a significant difference in the involvement of the CST between AQP4-negative and AQP4-positive patients. However, we did find that the number of brain lesions in the CST was significantly correlated with a higher EDSS in AQP4-negative patients, but not in those with AQP4 positivity. This suggests that in AQP4-negative patients, CST lesions may have a more pronounced impact on motor function, contributing to higher disability. It can be inferred that AQP4-negative patients may experience motor disabilities due to CST lesions, even if their spinal cord involvement is less severe or absent. In contrast, in AQP4-positive patients, the primary focus may be on managing spinal cord inflammation, as these lesions seem to be the main drivers of disability in this group.
To better understand this relationship, it would be valuable to consider specific components of the EDSS, such as the pyramidal score, which evaluates motor function. This could provide insights into whether motor deficits in AQP4-negative patients are more sensitive to the burden of CST lesions. The critical role of the CST in motor control and its involvement in the pathophysiology of NMO warrants further exploration.
The finding that hemispheric white matter lesions are linked to EDSS scores only in AQP4-positive patients, and not in AQP4-negative ones, highlights the distinct differences in lesion distribution, underlying mechanisms, and clinical implications between these groups. The AQP4 antibodies mainly target astrocytes, causing inflammatory damage in areas rich in AQP4, like the white matter tracts in the hemispheres (36). In AQP4-positive patients, these lesions are likely tied to greater inflammatory activity, which can moderately correlate with overall disease severity and EDSS scores. On the other hand, hemispheric white matter lesions in AQP4-negative patients may have less functional role or could even be incidental, not directly affecting disability. This indicates that while these lesions may indicate cumulative damage in AQP4-positive patients, in AQP4-negative patients, other lesions, such as those in the CST, might better account for disability progression, rendering hemispheric white matter lesions less clinically relevant in this group.
To achieve a clearer understanding of these relationships, advanced imaging techniques like diffusion tensor imaging (DTI) or volumetric analysis could be utilized to evaluate the structural integrity of white matter tracts and their link to EDSS, offering a more precise evaluation of disease severity.
The connection between the total number of brain lesions and the EDSS score in NMO emphasizes the crucial impact of brain involvement on the overall disability linked to this condition. This finding indicates that brain lesions in NMO are not just incidental; they play a significant role in the clinical disability faced by patients. The total burden of lesions can indicate the cumulative effects of the disease on both motor and non-motor pathways. Even if individual lesions do not directly affect motor functions, their collective impact can result in higher disability. For instance, lesions in motor-related regions like the CST can directly elevate EDSS scores, while lesions in other areas, such as the brainstem, cerebellum, or visual pathways, may indirectly influence functions like balance, coordination, and vision, all of which can lead to increased disability. Additionally, the accumulation of multiple small lesions, even in less critical areas, can contribute to cognitive decline, issues with gait and balance, and fatigue, further raising EDSS scores by impacting functional capacity.
A greater total lesion load may suggest more aggressive disease activity or widespread damage, such as astrocytic damage in AQP4-positive cases or oligodendrocyte insult in AQP4-negative/MOG-positive cases. This systemic inflammation affects various aspects of functioning, resulting in more significant disability, which is reflected in the EDSS. The strong relationship between lesion load and EDSS in both antibody populations emphasizes the significance of brain lesion monitoring in every NMO patient, independent of antibody status. It may highlight the need for more aggressive or tailored therapies for disease progression when the total lesion count is high. Furthermore, exploring whether the presence of an increasing number of brain lesions over time is associated with a steeper trajectory of EDSS could provide critical data to help address whether lesion burden can be implicated as a chronic contributor to disability.
There are, however, some limitations of this study that need to be considered. First, the dataset covers 13 years of data collected with conventional sequences, including T1-weighted, T2-weighted, FLAIR, and contrast-enhanced sequences. However, more advanced techniques such as DTI and MR spectroscopy (MRS) were not employed in all subjects, thus constraining the research. Second, our radiologic data were quite diverse. The images were acquired from different institutions in different cities, and different protocols were used. This lack of a standardized protocol led to variability in data quality, especially in diffusion-weighted sequences, which were missing for some participants. This limitation affected our capacity to analyze the diffusion characteristics of the brain lesions and their relation to the EDSS. Moreover, another limitation of this study is the retrospective design, which may introduce selection bias. Hence, future prospective studies with larger sample sizes and advanced imaging modalities, such as DTI, are warranted. These constraints show the need to enhance imaging protocols and employ new techniques in future research. Also, research that looks at the evolution of brain lesions over a longer period and how these changes are associated with alterations in EDSS may offer more information on the effects of brain lesions on disease progression.
In conclusion, our study demonstrates that EDSS is correlated with the total number of brain lesions in NMO, regardless of AQP4 status. Also, in AQP4-positive patients, contrary to AQP4-negative patients, spinal cord lesion length correlates with EDSS. Similarly, hemispheric white matter lesions were significantly associated with higher EDSS only in AQP4-positive patients. Exclusively in AQP4-negative patients, CST lesions are significantly correlated with disability. These findings highlight the importance of considering brain lesions in both AQP4-positive and negative NMO patients for assessing disease severity and disability. Furthermore, the differential impact of NMO lesions on EDSS in subgroups based on AQP4 antibody status suggests that prognostication and treatment strategies should be tailored to each group according to their AQP4 status. Future research should focus on longitudinal tracking of lesions to clarify the role of brain lesion evolution in disability progression and explore advanced imaging biomarkers and neuroimaging techniques (e.g., DTI and volumetric analysis) to refine prognostic models and personalized treatment strategies.