1. Context
Accurate diagnosis and timely treatment are crucial for managing pediatric patients with bone lesions, as misdiagnosis can lead to severe consequences. Bone infections, such as osteomyelitis, and primary bone sarcomas, like osteosarcoma and Ewing's sarcoma, often present with similar clinical features and radiological findings, posing significant diagnostic challenges for healthcare providers (1-3).Osteomyelitis is one of the most common musculoskeletal infections in children, with an estimated incidence of 8 - 10 cases per 100,000 children per year (4). In contrast, primary malignant bone tumors are significantly rarer, with osteosarcoma and Ewing’s sarcoma occurring at rates of approximately 3 - 5 cases per million children annually (5, 6). Osteomyelitis predominantly affects younger children, whereas osteosarcoma is most commonly diagnosed in adolescents, particularly during periods of rapid bone growth. This significant difference in prevalence underscores the importance of early and accurate differentiation to prevent unnecessary interventions or delayed diagnoses.
Misdiagnosis of bone sarcomas as infections can result in delayed referral to specialized centers, inappropriate treatment, and compromised patient outcomes (7). Conversely, mistaking bone infections for sarcomas may lead to unnecessary and aggressive interventions, such as chemotherapy, which can further weaken the patient's immune system and complicating their clinical course (8).
The rarity of pediatric bone sarcomas, along with their potential to mimic benign conditions or infections, contributes to the complexity of diagnosis (9). Symptoms of bone sarcomas, such as pain and swelling, can be mistaken for sports-related injuries, osteomyelitis, or growing pains, leading to delayed diagnosis and disease progression (10). Moreover, the histologic similarities between Ewing's sarcoma and other small blue round cell tumors require advanced diagnostic techniques, including immunohistochemistry and molecular genetics, to confirm an accurate diagnosis (11).
To address these diagnostic challenges and improve patient outcomes, a multidisciplinary approach to sarcoma treatment has emerged as a pivotal strategy (12). The integration of diverse specialties within a multidisciplinary team (MDT), including sarcoma surgeons, pathologists, oncologists, and radiation oncologists, allows for comprehensive evaluation, personalized treatment planning, and coordinated care throughout the patient's journey (13). Studies have demonstrated that patients with sarcomas managed by MDTs in high-volume centers exhibit improved survival rates and better treatment outcomes compared to those treated in low-volume settings (14).
This narrative review aimed to synthesize current evidence on the clinical presentations, diagnostic challenges, and multidisciplinary treatment approaches for distinguishing between bone infections and sarcomas in pediatric patients. By highlighting key findings from recent studies, we seek to provide guidance for healthcare providers in accurately differentiating these conditions, minimizing the risk of misdiagnosis, and optimizing patient care. Emphasis is placed on the importance of early and accurate diagnosis, the use of advanced diagnostic tools, and the implementation of multidisciplinary treatment strategies to improve outcomes and survival rates in pediatric patients with bone lesions.
2. Clinical Presentation and Diagnostic Challenges
Bone infections and sarcomas in pediatric patients often present with overlapping clinical features, making accurate diagnosis a challenging task for healthcare providers. Osteomyelitis, as a common bone infection, typically manifests with localized pain, swelling, erythema, and warmth at the affected site, accompanied by systemic symptoms such as fever and malaise (15). Similarly, primary bone sarcomas, including osteosarcoma and Ewing's sarcoma, present with persistent, progressive pain that worsens at night and is not alleviated by rest, along with the presence of a palpable mass or swelling (16). The non-specific nature of these symptoms, coupled with the rarity of pediatric bone sarcomas, contributes to the potential for misdiagnosis or delayed referral to specialized centers (17).
Moreover, the insidious onset and potential to mimic benign conditions further complicate the diagnostic process. Symptoms of bone sarcomas can be misinterpreted as sports-related injuries, growing pains, or other less severe conditions, leading to delayed recognition of the underlying malignancy (10). This diagnostic challenge highlights the critical need for early and accurate diagnosis to prevent disease progression, initiate appropriate treatment, and optimize patient outcomes.
3. Diagnostic Imaging and Differentiation
Diagnostic imaging plays a crucial role in distinguishing between bone infections and sarcomas in pediatric patients. Plain radiography, the initial imaging modality, can reveal distinct patterns that aid in differentiation. In cases of osteomyelitis, radiographs may show features such as cortical bone erosion, periosteal reaction, and soft tissue involvement, which can mimic tumorous osteolytic bone destruction (18). Conversely, sarcomas exhibit aggressive bone destruction, ill-defined margins, and intratumoral calcifications, reflecting their malignant nature (19).
Advanced imaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), provide additional insights into the extent of bone and soft tissue involvement. MRI is particularly useful in delineating the extent of infection, identifying abscesses, and differentiating osteomyelitis from cellulitis (20). In the context of sarcomas, MRI aids in assessing tumor extent, detecting recurrence, and evaluating treatment response (21).
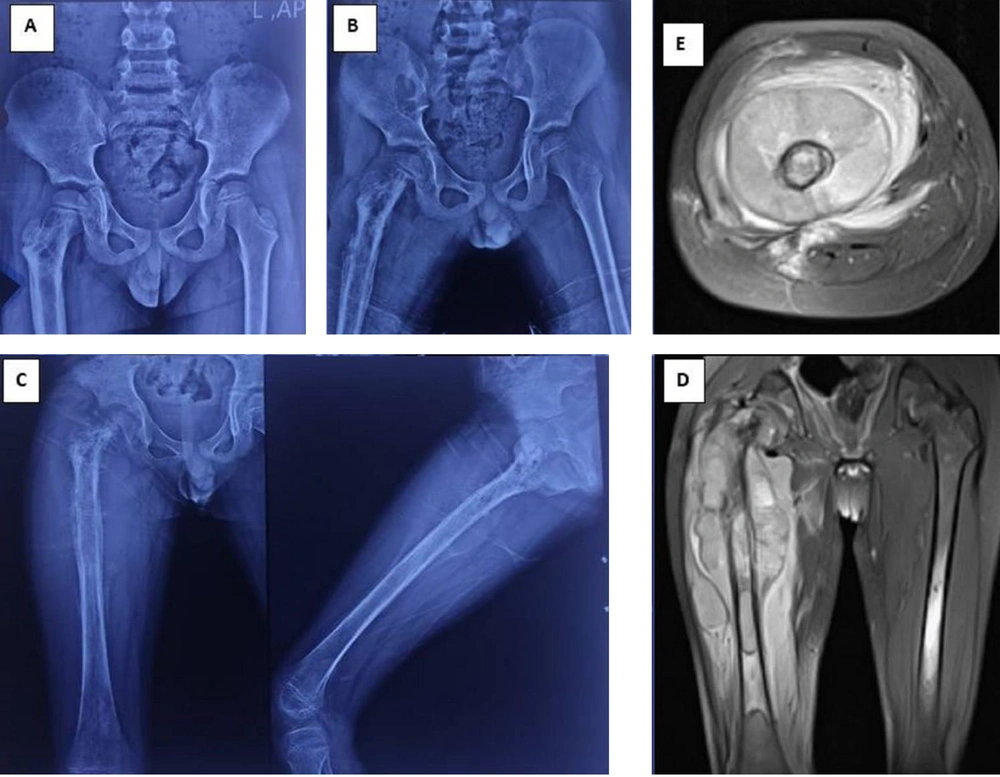
Despite the valuable information provided by imaging studies, distinguishing between bone infections and sarcomas based on radiological findings alone can be challenging. The aggressive appearance of some benign conditions, such as Rosai-Dorfman disease, may mimic infectious processes or malignant tumors, necessitating a comprehensive evaluation to avoid misinterpretation (22). Furthermore, the histologic similarities between Ewing's sarcoma and other small blue round cell tumors emphasize the importance of advanced diagnostic techniques, including immunohistochemistry and molecular genetics, to confirm the accurate diagnosis (23) (Figure 1).
Radiographic and magnetic resonance imaging (MRI) progression of a misdiagnosed case of Ewing's sarcoma. A, initial anteroposterior pelvic radiograph obtained 9 months prior to definitive diagnosis, demonstrating abnormalities in the proximal right femur. The patient presented with pain, limping, and low-grade fever, initially diagnosed and treated as acute osteomyelitis; B, follow-up radiograph 5 months prior to definitive diagnosis, showing disease progression despite symptomatic improvement. Surgical debridement was performed, with pathology suggesting infection, although cultures remained negative; C, radiograph at presentation to our tertiary center, revealing further progression of the lesion; D and E, coronal and axial MRI sequences, respectively, obtained at our center on the day of definitive diagnosis. Subsequent biopsy results confirmed Ewing's sarcoma.
4. Importance of Diagnostic Biomarkers in Differentiating Sarcoma from Infection
The differential diagnosis between bone sarcomas and infections, particularly osteomyelitis, remains a significant challenge in clinical practice. This distinction is crucial for appropriate patient management, as the treatment approaches for these conditions differ substantially. Recent advancements in biomarker research have opened new avenues for improving diagnostic accuracy and potentially reducing the need for invasive procedures.
4.1. Traditional Serum Biomarkers
Conventional serum biomarkers have long been used in the initial evaluation of bone lesions. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are typically elevated in both conditions but tend to be significantly higher in osteomyelitis compared to osteosarcoma. A study by Sigmund et al. (2020) found that CRP levels above 100 mg/L were more indicative of infection than malignancy (24). However, these markers lack specificity and can be elevated in various inflammatory conditions.
Alkaline phosphatase (ALP) is often elevated in osteosarcoma but not typically in infection, making it a useful adjunct in the diagnostic evaluation. Lactate dehydrogenase (LDH) is another enzyme that may be elevated in osteosarcoma, particularly in more advanced stages, but is less commonly increased in osteomyelitis (25).
4.2. Molecular Biomarkers and Liquid Biopsy
The advent of molecular techniques has revolutionized the field of cancer diagnostics, including bone sarcomas. Liquid biopsy, which involves the analysis of circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs), has emerged as a promising non-invasive diagnostic tool.
For Ewing sarcoma, the detection of the characteristic EWS-FLI1 fusion transcript in blood samples has shown potential as a diagnostic and monitoring tool. Aran et al. reported that ctDNA analysis could detect this fusion in up to 80% of Ewing sarcoma cases, offering a less invasive alternative to traditional biopsy (26).
In osteosarcoma, while no single genetic alteration is pathognomonic, several recurrent mutations have been identified. These include alterations in the TP53, RB1, and ATRX genes. The detection of these mutations in ctDNA could potentially aid in early diagnosis and treatment monitoring (27).
4.3. Inflammatory Markers and Cytokines
The inflammatory response in osteomyelitis and bone sarcomas differs, providing an opportunity for differential diagnosis. Procalcitonin (PCT) has been shown to be more specific for bacterial infections compared to malignancies. A study by Massaccesi et al. (2022) found that PCT levels were significantly higher in osteomyelitis compared to bone tumors (28).
Cytokine profiles also differ between these conditions. Interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are typically elevated in both conditions but tend to be higher in osteomyelitis. In contrast, vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) are often more elevated in sarcomas, reflecting their role in tumor angiogenesis and invasion (29, 30).
4.4. Novel Biomarkers and Future Directions
Emerging research is focusing on identifying novel biomarkers that could further improve diagnostic accuracy. MicroRNAs (miRNAs) have shown promise in this regard. Specific miRNA signatures have been associated with different sarcoma subtypes and could potentially be detected in blood samples (26).
Proteomics approaches are also being explored. Mass spectrometry-based techniques have identified protein signatures that may distinguish between osteomyelitis and bone sarcomas. For instance, S100A8 and S100A9 proteins have been found to be overexpressed in osteomyelitis compared to osteosarcoma (31).
4.5. Multipara Meter Diagnostic Algorithms
Given the complexity of differentiating between bone sarcomas and infections, researchers are increasingly developing multiparameter diagnostic algorithms that combine various biomarkers with clinical and imaging data. These algorithms aim to improve diagnostic accuracy by integrating multiple lines of evidence.
A study by Sigmund et al. (2020) proposed a diagnostic algorithm combining CRP, ALP, and specific imaging features, which showed improved accuracy in distinguishing osteomyelitis from bone sarcomas compared to individual parameters alone (24).
4.6. Challenges and Future Perspectives
While biomarker research has made significant strides, several challenges remain. The rarity of bone sarcomas makes large-scale studies difficult, and the heterogeneity of these tumors complicates the identification of universal biomarkers. Additionally, the overlap in inflammatory responses between infections and malignancies can lead to false-positive results.
In summary, while no single biomarker can definitively distinguish between bone sarcomas and infections in all cases, the combination of traditional serum markers, molecular biomarkers, and novel techniques offers a promising approach to improving diagnostic accuracy. As our understanding of the molecular basis of these conditions grows, we can expect further refinements in diagnostic strategies, ultimately leading to earlier and more accurate diagnoses, and improved patient outcomes.
5. Common Bone Infections Mimicking Sarcomas
Several bone infections can present with clinical and radiological features that resemble those of sarcomas, leading to diagnostic challenges in pediatric patients. These infections include.
5.1. Acute Hematogenous Osteomyelitis
The acute hematogenous osteomyelitis (AHO) is a common invasive bacterial infection in children, primarily affecting the metaphysis of long bones. Patients present with acute onset of fever, localized bone pain, swelling, erythema, and warmth, along with irritability and decreased range of motion in the affected limb (32). Laboratory findings include elevated inflammatory markers (ESR, CRP) and leukocytosis. Radiological features in the early stages consist of soft tissue swelling, periosteal thickening, and loss of fat planes, while later stages demonstrate lytic lesions, bone destruction, and periosteal reaction. The MRI exhibits high sensitivity in detecting early osteomyelitis, marrow edema, and soft tissue involvement (33). Treatment involves empiric intravenous antibiotics targeting common pathogens (e.g., Staphylococcus aureus), with a transition to oral antibiotics based on clinical response and culture results (34). Surgical intervention may be necessary for abscess drainage or debridement of necrotic tissue (35).
5.2. Chronic Recurrent Multifocal Osteomyelitis
The chronic recurrent multifocal osteomyelitis (CRMO) is a rare auto inflammatory bone disorder characterized by recurrent episodes of sterile osteomyelitis (36). Patients experience insidious onset of bone pain, swelling, and tenderness at multiple sites, with periods of remission between episodes (37). The CRMO may be associated with other auto inflammatory conditions, such as psoriasis or inflammatory bowel diseases (36). Radiological findings include osteolytic lesions, sclerosis, and hyperostosis on plain radiographs, while MRI demonstrates multifocal bone lesions with marrow edema and soft tissue inflammation. Whole-body MRI is useful in assessing the extent of disease and monitoring treatment response. Treatment options include nonsteroidal anti-inflammatory drugs (NSAIDs) as first-line therapy, with corticosteroids, methotrexate, or TNF-α inhibitors reserved for refractory cases. Bisphosphonates may be used for pain control and prevention of bone resorption (38).
5.3. Chronic Nonbacterial Osteomyelitis
The chronic nonbacterial osteomyelitis (CNO) shares many features with CRMO, presenting with insidious onset of bone pain and swelling, most commonly in the metaphyses of long bones (39). Chronic nonbacterial osteomyelitis can involve a single site (unifocal) or multiple sites (multifocal) and is associated with elevated inflammatory markers and potential skin manifestations, such as palmoplantar pustulosis (40). Radiological findings on plain radiographs include osteolytic lesions, sclerosis, and periosteal reaction, while MRI demonstrates bone marrow edema, soft tissue inflammation, and periosteal thickening. Whole-body MRI is helpful in detecting asymptomatic lesions and monitoring disease activity (41). Treatment for CNO involves NSAIDs as initial therapy for pain relief and inflammation control, with corticosteroids, disease-modifying antirheumatic drugs (DMARDs), or biologic agents considered for refractory cases. Bisphosphonates may be used for pain management and prevention of bone loss (42).
5.4. Brodie's Abscess
Brodie's abscess is a type of subacute osteomyelitis that presents as a localized intraosseous abscess, more commonly affecting the metaphysis of long bones, particularly the tibia (43). Patients with a subacute onset of localized bone pain, swelling, and occasional systemic symptoms, accompanied by elevated inflammatory markers and potential leukocytosis. Radiological features on plain radiographs include a well-defined lytic lesion with surrounding sclerosis and a thin rim of periosteal reaction. The CT demonstrates a hypodense lesion with a sclerotic rim and potential cortical breach, while MRI shows a central abscess with peripheral enhancement and surrounding marrow edema (44, 45). Treatment involves prolonged antibiotic therapy targeting common pathogens (e.g., Staphylococcus aureus) and surgical drainage of the abscess with curettage of the lesion. Follow-up imaging is necessary to ensure complete resolution and absence of recurrence (45).
5.5. Tuberculous Osteomyelitis
Tuberculous osteomyelitis is a rare form of extra pulmonary tuberculosis that can affect the bones and joints, with an insidious onset of bone pain, swelling, and potential systemic symptoms such as fever and weight loss (46). It is more common in the vertebrae (Pott's disease) and large joints (e.g., hip, knee) and may be associated with a history of tuberculosis exposure or immunosuppression (47). Radiological findings on plain radiographs include osteolytic lesions, erosions, and paravertebral soft tissue shadows, while CT demonstrates bony destruction, sequestra, and soft tissue abscesses. The MRI shows marrow edema, soft tissue inflammation, and abscess formation (48). Treatment involves multidrug antituberculous therapy for an extended duration (6 - 12 months) and surgical intervention for spinal instability, neurological deficits, or large abscesses. Close monitoring is essential for assessing treatment response and potential complications, such as spinal cord compression (46).
5.6. Langerhans Cell Histiocytosis
The Langerhans cell histiocytosis (LCH) is a rare disorder characterized by the abnormal clonal proliferation of Langerhans-type dendritic cells. The disease can affect various organs or systems in the body, with the skeletal system being the most frequently involved in approximately 80% of patients (49). The LCH is categorized into three types: Single-system single-site (SS-s), single-system multiple-site (SS-m), and multisystem (MS) (50). Patients with LCH may present with localized pain, swelling, erythema, and warmth at the affected site, accompanied by systemic symptoms such as fever and malaise. The non-specific nature of these symptoms can lead to misdiagnosis or delayed recognition of the underlying condition.
Diagnostic imaging plays a crucial role in identifying skeletal involvement in LCH. Plain radiography can reveal lytic lesions, cortical erosion, and periosteal reaction in the affected bones (51). Advanced imaging techniques, such as CT and MRI, provide additional information about the extent of bone and soft tissue involvement (52). Treatment options for LCH depend on the extent and severity of the disease, ranging from observation and oral medication to systemic corticosteroids, curettage, bone grafting, and internal fixation (53). The study by Abdelaal et al. (2020) found that non-operative treatment can lead to adequate bone healing within a few months, and partial or complete remodeling of bone deformities can be observed without surgical correction. However, surgical intervention may be necessary when cervical spine involvement poses a risk of instability and subsequent neurological complications (49) (Table 1).
| Feature | AHO | CRMO | Brodie Abscess (Subacute Osteomyelitis) | Tuberculous Osteomyelitis | LCH |
|---|---|---|---|---|---|
| Age group | Children (esp. < 5 years) | Older children, adolescents | Older children, adolescents | Children, young adults (esp. endemic areas) | Children < 10 years |
| Onset and pain | Acute, rapid onset; severe localized pain | Gradual onset, intermittent pain, recurrent episodes | Subacute, localized pain, often nocturnal | Insidious onset, chronic dull pain | Insidious pain, persistent swelling |
| Systemic symptoms | High fever, malaise, toxic appearance | Mild or absent fever, often no systemic illness | Minimal or no systemic symptoms | Low-grade fever, weight loss, night sweats | No systemic symptoms (except in multisystem disease) |
| Local signs | Swelling, erythema, warmth, refusal to bear weight | Swelling, tenderness, multiple bone involvement | Localized swelling, but no severe erythema/warmth | Swelling, mild erythema, sinus tract formation in advanced cases | Firm swelling, sometimes painful; can be multifocal |
| Lab findings (ESR, CRP, WBC) | Markedly elevated ESR/CRP, leukocytosis; positive blood culture in ~ 50% | Moderately elevated ESR/CRP; no leukocytosis; negative cultures | Mildly elevated ESR/CRP; normal WBC; negative cultures | Moderate ESR/CRP elevation; negative routine cultures; positive TB tests | Mild ESR/CRP elevation; normal WBC; biopsy confirms diagnosis |
| Radiographic features | Lytic bone lesion, periosteal reaction, soft tissue swelling | Osteolytic lesions with sclerosis, periosteal thickening | Well-defined lytic lesion with sclerotic rim (geographic appearance) | Osteolytic lesions, bone destruction, minimal periosteal reaction | Lytic lesions with beveled edges, punched-out appearance |
| MRI characteristics | Marrow edema, rim-enhancing abscess, subperiosteal fluid collection | Multifocal marrow edema; no abscess; chronic inflammatory pattern | Centrally necrotic area, surrounding marrow edema, rim enhancement | Heterogeneous marrow changes, abscess formation, soft tissue involvement | Enhancing soft tissue mass, marrow edema; no periosteal reaction |
| Distinguishing features | Rapid onset; systemic illness; quick response to antibiotics; sequestrum in chronic cases | Sterile (non-infectious); often multifocal; association with autoimmune diseases | Classic sclerotic rim on X-ray; mimics low-grade bone tumors | Strong association with TB history; spine involvement common (Pott's disease) | Can mimic aggressive sarcomas; biopsy confirms histiocytic infiltration |
Comparison of Common Bone Infections Mimicking Sarcomas
6. Postoperative Complications and Monitoring
Pediatric patients undergoing wide resection of bone sarcomas frequently experience postoperative fever and systemic inflammatory response syndrome (SIRS), which can be mistaken for infectious complications (54). Distinguishing between normal physiological responses and potential infections in the postoperative setting is essential to guide appropriate management and avoid delays in adjuvant therapy.
Careful monitoring and the use of imaging modalities, such as sonography, are recommended if postoperative infection is suspected. Prompt identification and treatment of infectious complications are crucial to prevent adverse outcomes and ensure timely initiation of subsequent therapeutic interventions (55).
7. Multidisciplinary Approach to Sarcoma Treatment
The multidisciplinary approach to sarcoma treatment has emerged as a cornerstone of improved patient outcomes and survival rates. The collaboration of diverse healthcare professionals within a MDT, including sarcoma surgeons, pathologists, oncologists, and radiotherapists, enables comprehensive evaluation, personalized treatment planning, and coordinated care throughout the patient's journey (56, 57).
Centralized management of sarcomas in specialized centers with MDTs has been associated with enhanced treatment efficacy, reduced costs, and improved overall patient outcomes (14). The expertise and collective decision-making of MDTs facilitate optimal local disease control, tailored treatment strategies, and seamless coordination of multimodal therapies, ultimately leading to better survival rates and quality of life for patients with sarcomas (58, 59).
8. Prognostic Factors and Survival Outcomes
Accurate diagnosis and timely treatment initiation are key prognostic factors in pediatric patients with bone sarcomas. Delayed diagnosis and inappropriate management due to misdiagnosis can lead to disease progression, metastasis, and compromised therapeutic responses, ultimately impacting survival outcomes (17).
Age at diagnosis, tumor size, and the presence of metastases also play significant roles in determining patient prognosis. Younger children, particularly those under 10 years of age, have been shown to have better overall survival rates in Ewing's sarcoma, highlighting the importance of early diagnosis and tailored treatment approaches for different age groups (60).
Interestingly, recent research suggests a potential positive association between postoperative infections and survival in sarcoma patients without metastasis (55). While further investigation is needed to understand the underlying mechanisms, this finding underscores the complex interplay between host immune responses and tumor biology in the context of sarcoma treatment.
9. Future Directions and Recommendations
This narrative review highlights the challenges and strategies in distinguishing between bone infections and sarcomas in pediatric patients. To improve diagnostic accuracy and minimize the risk of misdiagnosis, healthcare providers should adopt a comprehensive approach that integrates clinical presentation, biochemical markers, radiological findings, and biopsy results (8).
Advanced diagnostic tools, such as FDG PET-CT, have shown superior accuracy in detecting bone metastasis in children with Ewing's sarcoma compared to traditional bone scans, emphasizing the potential for improved diagnostic precision (61). Further research is needed to refine diagnostic strategies, develop novel biomarkers, and optimize multidisciplinary treatment approaches to enhance patient outcomes.
Implementing a multidisciplinary approach in sarcoma management, with coordinated efforts from oncologists, radiologists, pathologists, and orthopedic surgeons is essential for ensuring comprehensive care and improving survival rates (62). Centralization of sarcoma treatment in specialized centers with MDTs should be encouraged to provide patients with access to expert care and cutting-edge therapeutic options (63).
This narrative review has several limitations. First, the included studies vary in methodology, sample size, and diagnostic criteria, which may introduce heterogeneity in findings. Additionally, some studies relied on retrospective data or case series, which limits generalizability. Another limitation is the lack of large, prospective studies directly comparing imaging and biomarker effectiveness in differentiating infections from sarcomas. Finally, while we aimed to provide a comprehensive review, publication bias and the evolving nature of diagnostic techniques may impact the completeness of this analysis.
10. Conclusions
Distinguishing pediatric bone infections from sarcomas remains a significant diagnostic challenge due to overlapping clinical and imaging features. Timely and accurate diagnosis is crucial to prevent delays in appropriate treatment. This review emphasizes key differentiating factors, including clinical presentation, radiographic findings, and advanced biomarkers.
A multidisciplinary approach involving radiologists, pathologists, and oncologists plays a vital role in improving diagnostic accuracy and treatment outcomes. Future research should focus on refining diagnostic algorithms and developing novel biomarkers to further enhance early detection and minimize misdiagnosis. Centralizing care in specialized centers and fostering collaboration among specialists will ensure optimal patient management and improved prognoses.