1. Context
Environmental pollution caused by the release of toxic substances from the textile industry has become a significant concern (1, 2). Synthetic materials, particularly in the dyeing process, are favored for their ease of use and durability (3-6). However, this has led to increased scrutiny due to their environmental impact. Color fastness, defined as the ability of a material to retain its color over time, is influenced by factors such as lighting, washing, and rubbing (7, 8).
The side effects and damages caused by synthetic chemicals, including synthetic drugs, chemical poisons, synthetic additives, preservatives, and organic dyes, have been linked to various cancers and diseases. These chemicals also pose environmental risks, contributing to the gradual degradation of ecosystems. In recent years, there has been a shift towards replacing synthetic colors with natural pigments due to their lower toxicity and environmental compatibility (9).
Numerous plants worldwide serve as sources of natural dyes. While only a few are used industrially, many are employed traditionally, and some have been the subject of recent studies. The coloring properties of plants are attributed to compounds found in various plant parts, each with distinct chemical structures and extraction methods (10). A wide range of colors, including blue, red, yellow, white, black, and brown, can be derived from plants, with pigments present in roots, bark, leaves, fruits, and flowers (11).
Zabii dyes, derived from plants, are particularly valued for their non-toxicity, biodegradability, and medicinal properties. These dyes do not contribute to pollution or compromise drinking water quality. The demand for natural colors has increased due to their environmental compatibility and ability to return to nature. Chemically, most coloring compounds extracted from plants contain phenolic components, with flavonoids representing a significant category (11).
In recent years, the dyeing of textiles with vegetable dyes from leaves, flowers, fruits, bark, and roots has gained attention. The unique characteristics of textiles dyed with natural dyes — such as environmental compatibility, reduced toxicity, and antimicrobial, anti-allergy, anti-odor, and anti-cancer properties — underscore the importance of these renewable resources (12, 13).
2. Objectives
The present study aimed to synthesize the findings from the latest published articles on the natural extraction and application of dyes and antimicrobials as effective dyes and antibacterial agents in textiles. Various extraction methods for natural and antimicrobial dyes are reviewed, along with examples of their early applications in textile processing. Additionally, the properties of these dyes are discussed, and their advantages and disadvantages are examined. The primary objective of this study is to review the antimicrobial properties of dye-producing plants.
3. Methods
In this study, valid scientific articles indexed in the information banks of ISI, SID, PubMed, PubMed Central, Scopus, Web of Science using the keywords dyes, wool, anti-bacterial activity, medicinal plants in the years 2009 - 2020 are examined was placed
4. Results
4.1. Eucalyptus Dye
The essential oil of the eucalyptus medicinal plant is highly valued in the medical industry, perfumery, and for producing rose color (14). This plant possesses antimicrobial, anti-inflammatory, anti-cancer, and antiseptic properties (15, 16). A study demonstrated that eucalyptus leaf extract is effective for rose dyeing of cotton and wool fabrics (15). It was noted that lower temperatures enhance the rose dyeing process (17), whereas for chitosan cotton fabrics, higher temperatures improve the rose color (18).
4.2. Fabaceae (Acacia catechu Willd)
This medicinal plant is entirely utilized for its therapeutic properties (19). It exhibits antioxidant properties (20) and is effective in relieving sore throat, cough, hoarseness, and tonsillitis (21). Externally, its paste is beneficial for skin diseases and wounds (22). A boiled bath with this plant is an effective remedy for various skin ailments. In conditions like stomatitis, halitosis, tooth decay, and cavities, Acacia catechu, known as Khedira in Sanskrit, is beneficial due to its ability to deplete Kapha Dosha, dry mucous secretions, and restore taste. The plant extract has multiple medicinal effects, including antipyretic, anti-inflammatory, antidiarrheal, hypoglycemic, hepatoprotective, antioxidant, and antimicrobial activities (23, 24).
4.3. Shikakai (Acacia concinna)
A medicinal plant from the Acaciaceae family, Shikakai contains 3000 types of chemical and aromatic compounds. It grows as a common tree in Indian forests (25). The plant is rich in saponin, which contributes to its foaming properties (26, 27) and is also used in sperm removal processes (28).
4.4. Sour Tea (Hibiscus rosa-sinensis)
This medicinal plant, available as a shrub and tree, includes 250 species (29). It possesses strong antioxidant (30), anti-inflammatory (31, 32), antidiabetic, and anticancer properties (33). Phytochemical analysis reveals compounds such as quercetin glycoside, riboflavin, niacin, anthocyanin, anthocyanidin, malvalic acid, gentisic acid, and lauric acid.
4.5. Indigo (Indigofera tinctoria)
This plant produces a blue dye and is effective against bacteria such as Escherichia coli and Staphylococcus aureus. Native to tropical regions of Asia and Africa, indigo is now cultivated worldwide.
4.6. Rubia cordifolia
Known for producing the red dye madder, extracts from this Mediterranean climbing vine are effective against various bacteria and fungi.
4.7. Turmeric (Curcuma longa)
Commonly known as turmeric, C. longa produces the yellow spice/dye curcumin, which has antimicrobial properties. It is native to Southeast Asia and widely cultivated in India and other tropical regions.
4.8. Pomegranate (Punica granatum)
The peels of pomegranates are a good source of red dye and contain punicalagins with antimicrobial properties. The pomegranate is native to western Asia and the eastern Mediterranean.
4.9. Barberry (Berberis vulgaris)
The roots, bark, and berries of barberry produce a yellow dye. Berberine, found in barberry, has antimicrobial properties. This shrub is native to Europe, western Asia, and northern Africa but is now naturalized in many regions.
4.10. Woad (Isatis tinctoria)
Woad produces a blue dye, with compounds effective against some fungi and bacteria. It is native to Eurasia and has been cultivated in Europe and Asia for centuries.
4.11. Oak Galls (Quercus infectoria)
Oak galls, growths on oak trees caused by wasps, contain high levels of tannins with antimicrobial properties. They can be used to produce black or brown dye.
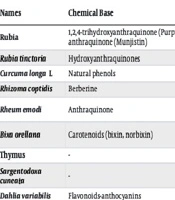
Table 1 lists a number of medicinal plants and active ingredients that have color-forming properties.
| Names | Chemical Base | Shade on Substrate | Substrate Applied | References |
|---|---|---|---|---|
| Rubia | 1,2,4-trihydroxyanthraquinone (Purpurin), 1,3-dihydroxy-2- carboxy anthraquinone (Munjistin) | - | Nylon with mordant | (34) |
| Rubia tinctoria | Hydroxyanthraquinones | Red | Wool with mordant | (35) |
| Curcuma longa L | Natural phenols | Yellow | Wool | (36) |
| Rhizomacoptidis | Berberine | Brownish yellow | Wool with mordant | (37) |
| Rheum emodi | Anthraquinone | Yellow, olive green | Wool, silk with metallic, mordants | (38) |
| Bixa orellana | Carotenoids (bixin, norbixin) | Yellow, orange, brown | Wool, silk color with metallic mordants | (39) |
| Thymus | - | - | Cotton | (40) |
| Sargentodoxa cuneata | - | - | Wool with mordant | (41) |
| Dahlia variabilis | Flavonoids-anthocyanins | - | Wool with mordant | (42) |
Pigment/Antimicrobial Properties Extracted from Different Parts of Plants and Their Application Properties
5. Discussion
Despite the various advantages of natural and antimicrobial dyes, there are limitations that restrict the use of these natural materials (43, 44). In the following sections, we will discuss these limitations. The interest in using medicinal plants stems from several reasons: Natural dyes with antimicrobial properties are inexpensive and renewable resources. A wide range of colors can be produced with a single natural dye or a mixture of several colors. Some natural resins possess inherent properties such as antimicrobial, insecticidal, anti-allergenic, and anti-ultraviolet effects.
In another study, it was reported that the oils of Bidens tripartita roots, B. tripartita L., Celosia argentea L. var. plumosa, Rubus fruticosus L., Indigofera heterantha Wall., Rubia cordifolia L., Bistorta amplexicaulis D. Don., Calendula officinalis L., Amaranthus hybridus L., Prunella vulgaris L., and Fragaria nubicola Lindl exhibit strong antimicrobial activity (45, 46). These plants contain active compounds such as flavonoids, alkaloids, sterols, and tannins, which contribute to their antimicrobial properties (47, 48). Another study highlighted the significant antimicrobial and chromogenic activity of the aqueous extract of Indigofera dendroides against various bacterial species (49).
5.1. Conclusions
As awareness of the high risks associated with synthetic dyes in textiles increases, there is a growing interest in the natural use of plant extracts as dyes and antimicrobial agents. These colors are extracted from different parts of plants, such as bark, leaves, roots, seeds, fruits, and flowers, which contain colored substances. The study results indicate that medicinal plants with coloring properties can be effectively used in the textile industry, paving the way for further investigations.