1. Context
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of reproductive age and has a long-lasting impact on health and fertility (1). This multifaceted syndrome is characterized by a spectrum of symptoms, including hormonal imbalance, ovarian dysfunction, hyperandrogenism, insulin resistance, and irregular menstrual cycles (2). Despite its significant impact on women's health and fertility, the exact etiology of PCOS remains elusive, necessitating the development of animal models that can precisely mimic its phenotype (3, 4).
In the quest to better understand PCOS and explore potential therapeutic interventions, the establishment of animal models that closely mimic the condition becomes imperative. Among the various animal models employed, rats have emerged as a particularly valuable species due to their physiological similarities with humans and ease of handling in laboratory settings (5). Various induction methods have been utilized to induce PCOS-like conditions in rat models, including hormone administration, genetic manipulation, and environmental perturbations (4, 5).
Among the multitude of induction methods, letrozole (LET), a potent aromatase inhibitor, has garnered significant attention for its efficacy in inducing PCOS-like phenotypes in rat models. LET's ability to disrupt the delicate hormonal balance, particularly by inhibiting the conversion of androgens to estrogens, closely mirrors the hormonal dysregulation observed in PCOS (4, 6). Understanding the mechanisms underlying LET induction and the resulting phenotypes is pivotal for elucidating the pathophysiology of PCOS and developing targeted therapeutic strategies.
2. Objectives
This review aims to provide a comprehensive examination of LET as an induction agent for PCOS models in rats. By delving into the mechanisms by which LET exerts its effects and the phenotypic manifestations it elicits, we can gain invaluable insights into the pathogenesis of PCOS. Furthermore, elucidating the nuances of LET-induced PCOS models will not only advance our understanding of the syndrome but also pave the way for the development of novel therapeutic interventions aimed at alleviating its burden on women's health.
3. Evidence Acquisition
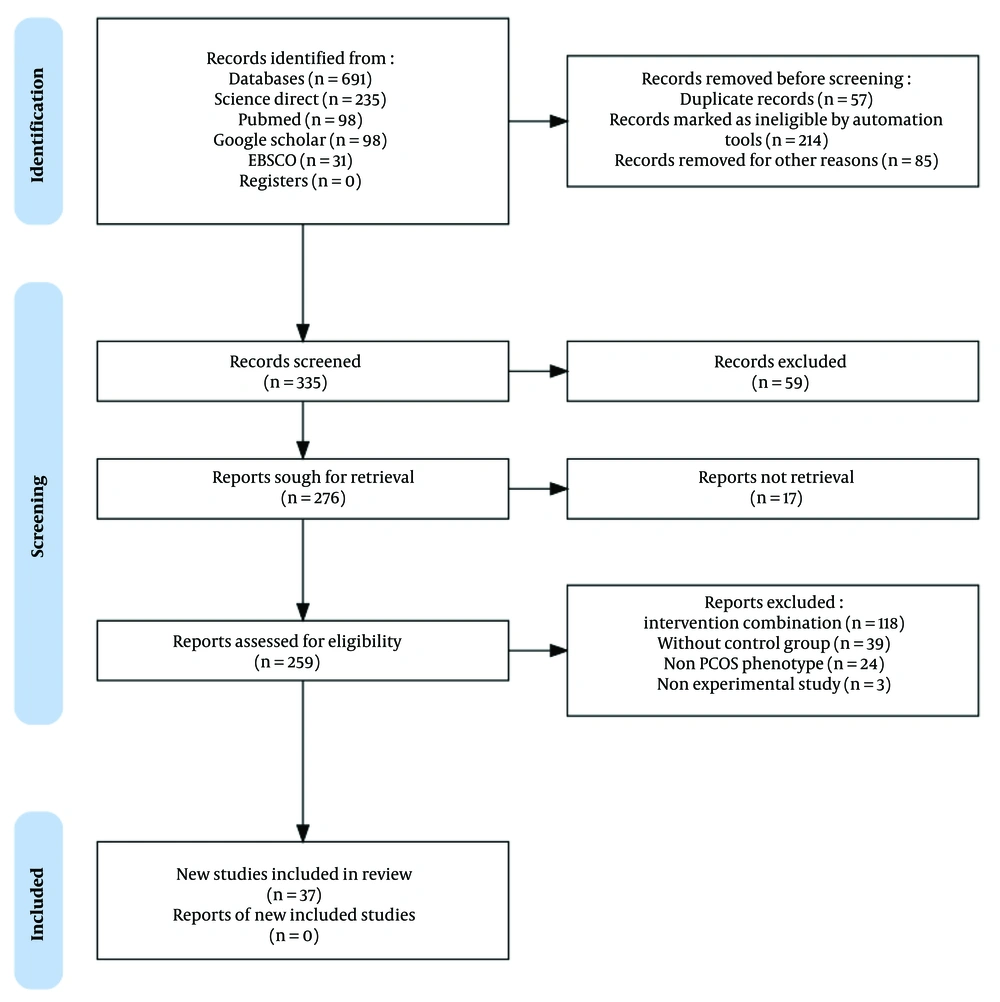
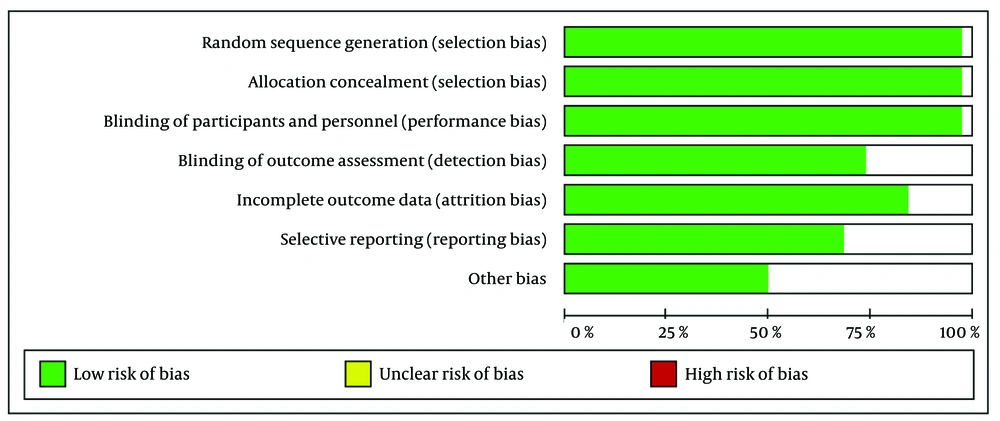
We conducted a comprehensive search of research articles published between 2020 and 2023, utilizing PubMed, ScienceDirect, EMBASE, and Google Scholar. Keywords aligned with Medical Subject Headings (MeSH) were employed, covering topics such as "animal model", "experimental animal", "laboratory animal model", "PCOS" and "letrozole", following PICOT criteria as outlined in Table 1. The research selected for the review is experimental animal research with a comparative design involving a control group. The allocation of animals into groups was carried out randomly (randomized allocation), with the application of blinding methods for both animals and researchers involved in data measurement and analysis. The sample size was based on the ARRIVE guidelines (animal research: Reporting of in vivo experiments) and OECD guidelines for animal studies (6 - 10 animals per group). Letrozole used in the study, in accordance with laboratory research standards but not for human consumption, was obtained from suppliers that provide specifically for research purposes, such as Sigma-Aldrich, LKT Labs, Rasa Research, MA Research Chems, Pharmaffiliates, and USV. Evaluation of study quality adhered to Joanna Briggs Institute (JBI) critical assessment and PRISMA guidelines (7). Duplicate literature was identified and excluded, while the remaining articles underwent two-stage screening based on predetermined inclusion criteria, as presented in Figure 1. This process was conducted by both reviewers to ensure accuracy and minimize errors. Risk of bias was assessed using the RoB 2 tool (8) with RevMan 5.41 software, as depicted in Figures 2 and 3.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Rat model | In vitro |
| Intervention | LET | LET combination with other treated |
| Comparators | With control group | Without control group |
| Outcomes | Research shows PCOS phenotype | Does not form PCOS phenotypes |
| Time | Within the past five years | More than the past five years |
| Study design | Experimental research | Analytical observational research |
| Language | Indonesian, English | Besides Indonesian and English |
Inclusion and Exclusion Criteria
4. Results
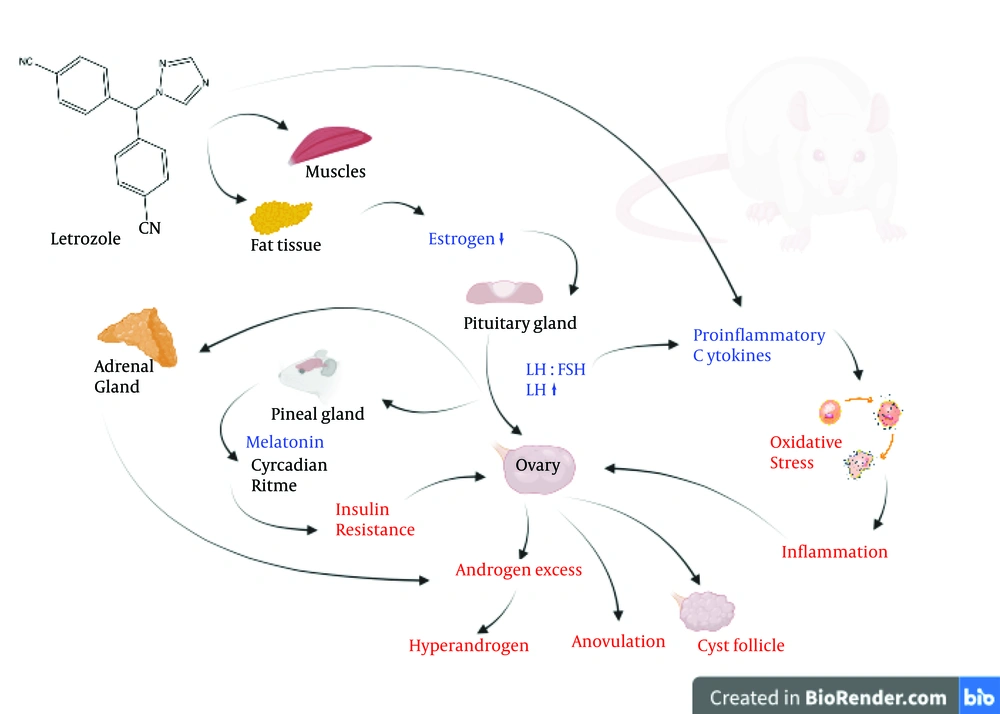
Thirty-seven research articles were included in this study. The LET induction dose, duration, method of administration, and PCOS phenotypes observed in the experimental models are presented in Table 2. The use of LET in the PCOS rat model aims to induce conditions that resemble the characteristics of PCOS in humans. The mechanism of LET induction in the PCOS rat model is through aromatase inhibition, leading to decreased estrogen production, increased luteinizing hormone (LH) and androgen levels, and disrupted hormonal feedback (Figure 4). LET functions as an aromatase inhibitor, an enzyme involved in the conversion of androgens to estrogen. By inhibiting aromatase, LET causes an overall decrease in estrogen production in the rat body (9, 10). The decrease in estrogen levels stimulates the release of LH from the pituitary gland. Reduced estrogen negative feedback on the pituitary gland can increase LH production (6, 10). Increased levels of LH can stimulate the ovaries to form antral follicles or cyst-like structures (11, 12). The antral follicles may become abnormal, and their increased numbers create a condition that resembles polycystic ovaries (6, 13, 14). Increased LH and hormonal imbalance can lead to ovulation disorders or anovulation (10, 15-19). The rat's menstrual cycle may become irregular or cease due to this hormonal disruption (20-24). Decreased estrogen production and increased LH may contribute to elevated androgen levels in the ovaries of rats (24-27). Increased androgens may play a role in the development of abnormal antral follicles (11, 23). Abnormal or non-ovulating antral follicles may develop into cyst-like structures (18, 28-30). These structures may reflect the characteristics of polycystic ovaries seen in women with PCOS. This mechanism creates an unbalanced hormonal condition and can lead to hormone patterns that resemble PCOS in humans (6, 31, 32).
| Dose | Duration | PCOS Phenotipe | Reference |
|---|---|---|---|
| LET 4.5 mg s.c implant | 90 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenicInsulin resistance | (6) |
| LET 50 μg s.c implant | 60 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic; inflammation | (14) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic; insulin resistance | (11) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; hyperandrogenic | (12) |
| LET 1 mg/kg p.o | 5 (wk) | Cystic ovary; irregular estrous cycle; hyperandrogenic; insulin resistance; inflammation | (35) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; inflammation | (22) |
| LET 1 mg/kg p.o | 28 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic | (10) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; inflammation | (36) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic | (27) |
| LET 6 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic; insulin resistance | (25) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic; insulin resistance | (28) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; hyperandrogenic; inflammation; insulin resistance | (9) |
| LET 35 mg/kg p.o | 4 (wk) | Cystic ovary; irregular estrous cycle; hyperandrogenic; insulin resistance | (37) |
| LET 1 mg/kg p.o | 21 (d) | Hyperandrogenic; inflammation; insulin resistance | (38) |
| LET 6 mg/kg p.o | 21 (d) | Irregular estrous cycle; hyperandrogenic; inflammation; insulin resistance | (33) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; hyperandrogenic; cystic ovary | (30) |
| LET 6 mg/kg p.o | 21 (d) | Cystic ovary; anovulation; hyperandrogenic; insulin resistance; inflammation | (34) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; hyperandrogenic; inflammation | (32) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; irregular estrous cycle; insulin resistance | (29) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; cystic ovary; hyperandrogenic | (39) |
| LET 1.8 mg/pellet s.c implant | 60 (d) | Arrest estrous cycle; hyperandrogenic; inflammation; cystic ovary | (40) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; cystic ovary; hyperandrogenic; insulin resistance | (21) |
| LET 1 mg/kg p.o | 21 (d) | Anovulation; hyperandrogenic; insulin resistance; inflammation; cystic ovary | (16) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; hyperandrogenic; insulin resistance; cystic ovary | (23) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; inflammation; hyperandrogenic; insulin resistance; cystic ovary | (20) |
| LET 1 mg/kg p.o | 21 (d) | Inflammation; hyperandrogenic; insulin resistance; anovulation | (15) |
| LET 400 μg/kg s.c implant | - | Anovulation; cystic ovary; hyperandrogenic; insulin resistance | (17) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; hyperandrogenic; insulin resistance | (41) |
| LET 1 mg/kg p.o | 28 (d) | Cystic ovary; hyperandrogenic; anovulation | (26) |
| LET 1 mg/kg p.o | 21 (d) | Irregular estrous cycle; cystic ovary; anovulation; hyperandrogenic; insulin resistance | (42) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; anovulation | (18) |
| LET 3 mg/kg p.o | 40 (d) | Cystic ovary; hyperandrogenic; insulin resistance; inflammation | (43) |
| LET 1 mg/kg p.o | 3 (wk) | Hyperandrogenic; insulin resistance; inflammation; multiple cysts | (31) |
| LET 1 mg/kg p.o | 21 (d) | Cystic ovary; hyperandrogenic; anovulation; inflammation | (19) |
| LET 1 mg/kg p.o | 21 (d) | Cystic follicle; insulin resistance | (44) |
| LET 1 mg/kg p.o | 3 (wk) | Anovulation; cystic follicle; hyperandrogenic; insulin resistance; microbiota-inflammation | (45) |
| LET 50 μg s.c implant | 60 (d) | Irregular estrous cycle; hyperandrogenic | (24) |
Phenotypic Features of Polycystic Ovary Syndrome in Letrozol-Induced Mice
Letrozole consistently produces various phenotypes similar to PCOS in humans, such as ovarian cysticity, irregular estrous cycles, hyperandrogenism, insulin resistance, and inflammation (25, 33, 34). This reflects the complexity of the PCOS condition involving multiple hormonal and pathophysiological aspects (13, 14). The use of LET as an induction agent for the PCOS model in mice provides an advantage in preclinical research, producing a phenotype similar to human PCOS with good consistency. With the ability to induce a phenotype like PCOS, this model can be used to test the effectiveness of various potential therapies and to understand the biological basis of PCOS.
The LET-induced PCOS mouse model is a research approach to understanding the biological basis of PCOS and is not a perfect representation of the human condition. Nonetheless, this mouse model can provide insight into the hormonal and structural changes that occur in PCOS. Although the LET-induced PCOS model mimics several features of the human condition, there are notable differences between the rat models and human PCOS. These differences highlight the limitations of this model in fully replicating the complexity of human PCOS. Variations in hormonal regulation, ovarian structure, and metabolic responses between rats and humans may affect the translational value of the findings. Further refinement of the rat model could enhance its applicability in PCOS research. Suggestions for improvement include modifications to better replicate the hormonal and metabolic profiles observed in human PCOS. Exploring the use of combined models or alternative animal models may address some of the limitations of the current approach and improve the translational relevance of the findings. Future research should consider these refinements to optimize the utility of animal models in understanding PCOS pathophysiology and developing effective treatments.
5. Conclusions
The LET-induced PCOS mouse model replicates key features of human PCOS, including ovarian cyst formation, irregular estrous cycles, hyperandrogenism, insulin resistance, and inflammation. LET inhibits aromatase, reducing estrogen production, disrupting estrogen negative feedback, and increasing LH and androgen levels. While this model provides valuable insights into PCOS pathophysiology, it has limitations. Physiological and metabolic differences between rodents and humans may affect the translation of results. Additionally, it lacks certain human-specific factors, such as genetic and environmental influences on PCOS.
Despite these limitations, the LET-induced model remains a useful tool for studying PCOS mechanisms and testing potential therapies. Future research should refine the model to better mimic human PCOS by incorporating dietary and genetic factors. Complementary approaches, such as alternative animal models or in vitro systems, could enhance translational relevance. Recognizing these limitations while optimizing the model will improve its applicability in developing effective PCOS treatments.