1. Background
Intravitreal injections (IVIs) represent the most rapidly expanding procedure in both ophthalmology and medicine overall. The introduction of molecular treatments targeting vascular endothelial growth factor (VEGF) has significantly changed the visual prognosis and management of patients with diabetic macular edema (DME), retinal vein occlusion, age-related macular degeneration (AMD), and other conditions leading to choroidal neovascularization or retinal vascular leakage. However, these injections carry a slight risk of developing endophthalmitis. The post-injection endophthalmitis rate ranges between 0.02% and 1.6% (1, 2). Despite this low risk, the visual repercussions may be serious. Although coagulase-negative Staphylococcus species are the most frequently isolated pathogens in culture-positive cases, infections caused by Streptococcus species tend to result in poorer visual outcomes (3, 4). Furthermore, using prophylactic topical antibiotic agents may lead to the emergence of atypical pathogens resistant to standard antimicrobial treatments, with an increasing number of reports describing unusual microbes identified in intraocular fluids from patients who develop post-injection endophthalmitis (1).
Infectious endophthalmitis (IEO) is a serious form of intraocular inflammation that can lead to irreversible blindness if not treated promptly (2). While it is a rare complication of intraocular procedures, its implications can be significant. Reports indicate that 36.1% of cases develop long-term complications such as persistent vitreous debris, epiretinal membranes, macular edema, and retinal detachments, while 31.2% of patients experience poor visual outcomes of counting fingers or worse, even after treatment (3). Typically, endophthalmitis presents within three days following an injection, although cases can emerge as soon as one day post-injection or even several weeks later (1, 4).
Endophthalmitis is classified based on the timing of infection (chronic or acute), etiology (fungal or bacterial), transmission route (exogenous or endogenous), and the specific organisms involved (5, 6). As mentioned, endophthalmitis can originate from exogenous or endogenous sources, with exogenous cases being the most prevalent. Among these, ocular surgery is the main cause of endophthalmitis (6, 7). Endogenous endophthalmitis, which arises from infections elsewhere in the body and spreads through the bloodstream, is rare, accounting for an estimated 2% - 8% of all endophthalmitis cases (8).
Intravitreal anti-VEGF injections have become the standard treatment for conditions such as AMD, neovascular diabetic retinopathy (DR), and macular edema caused by retinal vein occlusion. However, despite the considerable therapeutic benefits observed over the years, complications have not been entirely eliminated (9). Infectious endophthalmitis remains one of the most feared complications associated with IVIs. Common symptoms of bacterial IEO include eye pain, rapid vision loss, hypopyon, conjunctival redness, and vitreous opacification (4). Bacterial infections account for the majority of these cases (10, 11).
The use of intravitreal anti-VEGF agents has surged recently, as evidenced by Medicare data indicating an increase from fewer than 3,000 IVIs in 2000 to about 1.3 million in 2009 and over 2.6 million in 2014 (12). Treatment protocols generally involve pars plana vitrectomy (PPV) for patients with light perception (LP) visual acuity and intravitreal antibiotic therapy (IVAI) for those with better visual acuity than LP (9). Recent studies have demonstrated that early IVAI, followed by later PPV, may be an effective alternative treatment strategy, especially considering advancements in surgical techniques and equipment, which have led to improved outcomes (13). However, existing data on the best initial management strategies for post-injection endophthalmitis remain limited.
2. Objectives
We evaluated the role of intravitreal anti-VEGF injections in the overall incidence of IEO at our tertiary care referral center. Additionally, we characterized the microbial pathogens and clinical outcomes related to post-injection endophthalmitis to enhance control of this serious iatrogenic complication.
3. Methods
This retrospective study examined the medical records of patients from the Ophthalmology Department at Imam Khomeini Hospital, Ahvaz, Iran, between April 2021 and July 2024. All eyes that developed IEO after IVIs were included in the study. We defined post-IVI endophthalmitis as a case that raised sufficient clinical suspicion to warrant surgery, which could include vitreous tap and intravitreal antibiotic injection (“tap and inject”, or TAI) and/or PPV. All eyes suspected of having IEO received early PPV or TAI according to the physician’s assessment and clinical presentation.
A vitreous tap was performed using a 25- or 27-gauge needle to aspirate vitreous fluid, followed by the administration of intravitreal antibiotics. In some instances, based on clinical judgment, immediate PPV was performed along with IVI administration of antibiotics. The IVI protocol included ceftazidime (2 mg/0.1 mL) and vancomycin (1 mg/0.1 mL), along with dexamethasone (0.4 mg/0.1 mL) during TAI or PPV. Culture results and sensitivities were utilized to guide further IVAI.
Topical antibiotic drops were administered hourly to all patients, with the frequency reduced based on clinical improvement. As deemed necessary by the physician, fortified ceftazidime (50 mg/mL), fortified vancomycin (25 mg/mL), or moxifloxacin hydrochloride (0.5%) were provided. All patients received cycloplegic treatment with either topical atropine sulfate (0.5% drops) or cyclopentolate (2% drops). Topical steroids, such as prednisolone acetate (1%) or dexamethasone (0.1%), were prescribed in every case. Systemic steroids were administered at the doctor’s discretion, given daily at a dose of 0.5 - 1 mg/kg/day, and were gradually tapered based on clinical response over 6 to 8 weeks.
Patients were assessed daily, and as clinical improvement was noted, topical treatments were reduced, and follow-up intervals were lengthened. The main outcome assessed was best-corrected visual acuity (BCVA) at presentation and discharge. Secondary outcomes included microbiological characteristics, BCVA, post-IVI endophthalmitis, and clinical findings at presentation.
Medical record examination was conducted to detect patients with IEO within six weeks after IVI during the research period. The gathered data included demographic information, underlying conditions necessitating IVI therapy, treatment history (such as injection agents and the last IVI date), clinical observations from slit-lamp biomicroscopy at the time of endophthalmitis presentation (including anterior chamber cells, corneal edema, posterior synechiae, preretinal exudates, anterior chamber fibrin, vitritis, hypopyon, and intraretinal hemorrhages), initial procedures (PPV or therapeutic anterior chamber injection), subsequent procedures (PPV), systemic steroid therapy, topical antibiotic therapy, culture results, and the last recorded BCVA at follow-up and presentation at discharge. Best-corrected visual acuity was evaluated using Snellen charts.
The time intervals between the final IVI and the onset of symptoms, between the IVI and the initial procedure, and between the symptom onset and the first procedure were also documented. Infectious endophthalmitis was diagnosed based on clinical evaluation, and the initial management was decided by the examining ophthalmologist. In all endophthalmitis instances occurring after anti-VEGF injections, vitreous fluid was collected for microbial culture, and the findings were later analyzed. All categorical variables were summarized in terms of frequency and percentage. Data analysis was conducted using IBM SPSS Statistics software, version 25.0 (Armonk, NY: IBM Corp).
4. Results
A total of 7,396 IVIs were performed, resulting in 15 diagnosed cases of post-IVI IEO, all following bevacizumab injections. This resulted in an overall incidence of 0.2%. Most patients exhibited clinical symptoms between 3 and 5 days following the injection, including conjunctival congestion, vision loss, and inflammation in both the vitreous and anterior chamber, indicating acute endophthalmitis, which was present in all cases. Vitreous cultures were positive in 6 out of the 15 eyes examined, resulting in a positive culture rate of 40%. The most frequently isolated organism from these positive cultures was Enterococcus, which accounted for 50% of the cases. Enterobacter was isolated in 33.3% of the cases, and one instance of Staphylococcus aureus was also identified. Table 1 provides additional details on the distribution of these isolated organisms, while Table 2 presents demographic data for the patients.
| Patients | Sex | Underlying Disease | Medication | VA at Presentation | Day to Presentation | Treatment | Culture Result | Continued Anti-VEGF | Age | Final VA After One Month Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | DME | Bevacizumab | LP | POD4 | IVAB + DEX PPV + IV AB | No growth | IVB | 65 | HM |
| 2 | F | DME | Bevacizumab | FC = 30 cm | POD2 | IVAB + DEX + IV AB | No growth | IVB | 55 | 1/10 |
| 3 | F | DME | Bevacizumab | HM | POD14 | IVAB + DEX PPV + IV AB | Enterococci | IVB | 70 | HM |
| 4 | M | DME | Bevacizumab | FC = 50 cm | POD1 | IVAB + DEX + IV AB | No growth | IVB | 60 | FC = 6 m |
| 5 | F | DME | Bevacizumab | HM | POD8 | IVAB + DEX + IV AB | Enterobacter cloacae | IVB | 60 | FC = 30 cm |
| 6 | F | DME | Bevacizumab | HM | POD6 | IVAB + DEX + IV AB | E. cloacae | IVB | 68 | FC = 60 cm |
| 7 | M | DME + AGV | Bevacizumab | HM | POD1 | IVAB + DEX + IV AB | Enterococci | IVB | 70 | HM |
| 8 | M | DME | Bevacizumab | FC = 15 cm | POD3 | IVAB + DEX PPV + IV AB | No growth | IVB | 60 | FC = 4 m |
| 9 | M | DME | Bevacizumab | HM | POD5 | IVAB + DEX + IV AB | No growth | IVB | 45 | FC = 3 m |
| 10 | M | DME + HRCPDR | Bevacizumab | LP | POD3 | IVAB + DEX PPV + IV AB | No growth | IVB | 65 | HM |
| 11 | F | DME | Bevacizumab | HM | POD3 | IVAB + DEX + IV AB | No growth | IVB | 71 | FC = 1.5 m |
| 12 | F | DME | Bevacizumab | HM | POD25 | IVAB + DEX + IV AB | Enterococci | IVB | 61 | FC = 20 cm |
| 13 | F | DME | Bevacizumab | HM | POD2 | IVAB + DEX PPV + IV AB | No growth | IVB | 56 | HM |
| 14 | F | DME | Bevacizumab | FC: 30 cm | POD5 | IVAB + DEX + IV AB | No growth | IVB | 80 | FC: 1 m |
| 15 | M | AMD | Bevacizumab | HM | POD5 | IVAB + DEX + PPV + IV AB | Staphylococcus aureus | IVB | 30 | HM |
Summary of a Patient with Endophthalmitis After Anti Vascular Endothelial Growth Factor Agent Intravitreal Injection
| Variables | Values |
|---|---|
| Sex | |
| Female | 8 (53.3) |
| Male | 7 (46.6) |
| Age (y) [median (range)] | 57.5 (35 - 80) |
| Indication for IVI | |
| AMD | 1 (6.6) |
| DME | 14 (93.3) |
| Clinical presentation | |
| Symptoms | |
| Decrease in vision | 15 (100) |
| Pain | 10 (66.6) |
| Redness | 15 (100) |
| Signs | |
| Corneal edema | 15 (100) |
| Anterior chamber cell | 15 (100) |
| Hypopyon | 10 (56.6) |
| Posterior synechiae | 0 (0) |
| Hypopyon | 15 (100) |
| Culture | |
| Positive | 6 (40) |
| Negative | 9 (60) |
| Pathogen | |
| Enterococci | 3 (50) |
| Enterobacter | 2 (33.3) |
| Coagulase-negative staphylococci | 1 (16.6) |
| Management | |
| Systemic steroids | |
| Yes | 15 (100) |
| No | 0 (0) |
| Primary procedure | |
| TAI | 9 (60) |
| PPV + TAI | 1 (6.6) |
| Second procedure | |
| PPV | 5 (33.3) |
Demographics, Incidence, Laboratory Findings, Clinical Presentation, and Management of 15 Post-Intravitreal Injection Endophthalmitis Cases (N = 15) a
Among the 15 cases, 7 (46.7%) were male and 8 (53.3%) were female, with a median age of 57.5 years. Five cases (33.3%) involved the left eye, whereas 10 cases (66.7%) involved the right eye. The primary reasons for IVIs included DME in 93.3% of the cases and AMD in 6.7%. The most common symptom was decreased vision, reported by all 15 patients (100%). The most frequently observed clinical symptoms included vitritis and anterior chamber cells, which were present in all cases (100%).
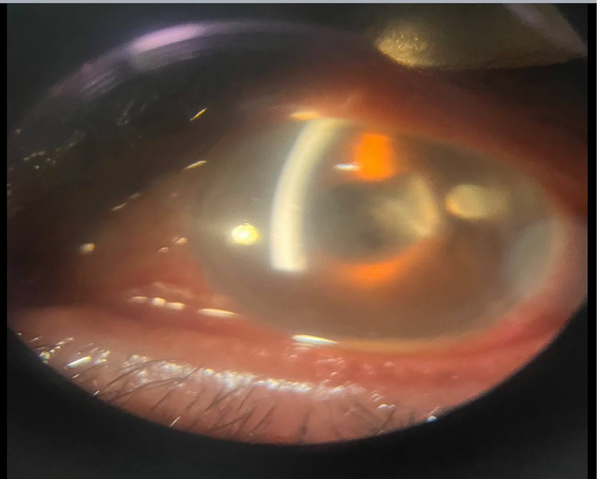
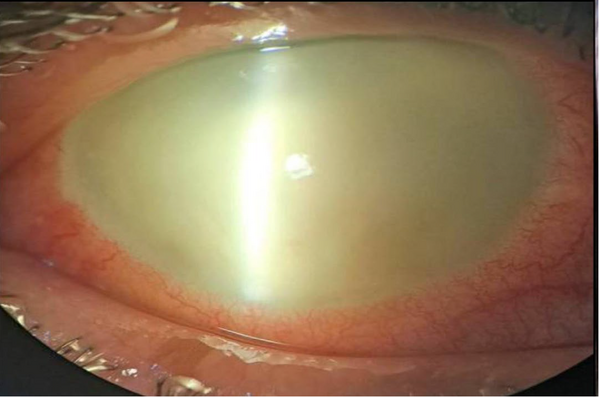
The first procedure conducted was tap and injection (TAI) in 9 (60%) of the patients, during which all received IVIs of ceftazidime (2 mg/0.1 mL), dexamethasone (0.4 mg/0.1 mL), and vancomycin (1 mg/0.1 mL), along with the collection of a vitreous sample. Among the remaining patients, one received TAI, dexamethasone, and PPV simultaneously (patient number 13). Additionally, 5 patients first underwent TAI and dexamethasone, followed by PPV (patients numbered 1, 3, 8, 10, and 15). In the cases of endophthalmitis, the TAI and dexamethasone group consisted of 9 (60%) patients. Conversely, the PPV group comprised 6 (40%) patients. Figures 1 and 2 show the acute-onset presentation of postoperative endophthalmitis and acute endophthalmitis, respectively.
5. Discussion
As VEGF antagonists have emerged as the primary therapy for prevalent retinal conditions characterized by retinal vascular leakage and neovascularization, such as retinal vein occlusions, DME, and neovascular AMD, there has been increasing concern about the risk of IEO following IVIs of these drugs. Although many large retrospective studies indicate that the incidence of IEO is relatively low (around 0.05% or five in 10,000 injections), the risk of iatrogenic infection rises for individual patients who may need repeated monthly injections. The use of topical antibiotics for prophylaxis before and after procedures has been debated, with recent evidence suggesting that they may not significantly improve visual outcomes (14, 15). The widespread application of prophylactic antibiotic agents can lead to the development of antibiotic-resistant organisms and increase the prevalence of treatment failure.
In the present retrospective case-series research, the incidence rate, microbiological and clinical characteristics, control strategies, and outcomes of post-IVI IEO were presented. Our results indicate that the overall incidence of post-IVI IEO was 0.2%. In a meta-analysis by Bande et al., rates of IEO after IVIs ranged between 0.012% and 0.10% (16). The findings of the current study show a higher incidence of IEO compared to previously published data. The most frequently reported symptom in our study was decreased vision, followed by pain and redness, which is consistent with earlier literature (17). The median time between IVI and the onset of symptoms was 5 days, aligning with incidences reported in previous studies (18, 19).
Among the cases of IEO following anti-VEGF injection, 40% tested positive in cultures. In our study, Enterococcus was identified as the causative organism in half of these cases, differing from earlier reports (8, 20). We also detected several other organisms, including Enterobacter cloacae and S. aureus. Enterobacter cloacae is a gram-negative commensal bacterium typically found in the human gastrointestinal tract and has been reported in endophthalmitis cases after trauma and cataract surgery (18, 19).
Concerning the demographic factors assessed, age did not influence the final visual outcome. Conversely, Davidov et al. assessed 23 cases with post-anti-VEGF IVI endophthalmitis and indicated that younger age was linked to more favorable visual outcomes (21). Our findings showed that better visual acuity at presentation was linked to improved visual outcomes, consistent with Davidov et al.’s report that baseline BCVA was associated with better visual results (21). A negative culture result was significantly associated with better visual outcomes, contrasting with the findings of Dossarp et al.’s study, potentially influenced by the specific pathogens in the culture-positive subgroup (1).
In our analysis, patients who received TAI had better outcomes than those who underwent PPV. While previous retrospective analyses indicate no significant differences in outcomes between patients receiving TAI or PPV, our research does not support this conclusion (22).
In cases of endophthalmitis, several possible risk factors could be suggested. First, the use of repackaged bevacizumab syringes may have contributed to the increase. Second, inconsistencies in refrigerator storage could have led to greater contamination of bevacizumab vials during the pooling process. Third, contact between the needle and the eyelashes or lid margins during the procedure may have been a contributing factor. Lastly, the involvement of in-training fellows might have influenced the higher incidence rates. These factors emphasize the need for strict adherence to protocols regarding medication handling, storage, and procedural techniques to reduce the risk of endophthalmitis.
However, certain limitations must be recognized. First, the retrospective design of the study presents inherent challenges, including potential selection bias and dependence on pre-existing data. Additionally, as the data comes from a single center, the findings may not be fully generalizable to a wider population. Variations in surgical proficiency among different surgeons could also influence the outcomes. Lastly, the relatively small number of endophthalmitis cases included may affect the statistical power and accuracy of the results. To strengthen future analyses, we plan to expand the sample size by incorporating data from multiple centers.
5.1. Conclusions
Infectious endophthalmitis following IVIs of anti-VEGF drugs is a rare yet severe complication of a procedure that has become routine in retinal practice. Due to the lack of established evidence-based guidelines, more information on the microorganisms responsible and the clinical progression of cases could help inform management strategies. Our findings can help predict outcomes and inform decision-making related to the treatment and diagnosis of post-injection IEO. Additional prospective trials are needed to develop comprehensive management guidelines for post-IVI endophthalmitis.